
The risk of developing acute non-lymphocytic leukemia in women with breast cancer
Introduction
Among women around the world, breast cancer is the malignancy most frequently diagnosed and is also the leading contributor to cancer-associated mortality among females (1). Attributed to early screening, advanced detection, and efficient treatment, breast cancer patients are now more likely to achieve long-term survival and better life expectancy. However, in their prolonged lifetime, survivors face the rising problem of second primary malignancies, which are independent tumors subsequent to the first breast tumor, which may be related to the shared etiology or side-effects of treatment (2). In comparison with the population generally, cancer patients have a heightened risk of developing a subsequent primary cancer, and around 10% of individuals with breast cancer go on to develop a second primary cancer, with second hematopoietic diseases as one example (3). Therefore, it is pivotal for breast cancer patients to manage subsequent primary problems (4).
Previous investigations have suggested a 30% excess risk of second primary tumors, excluding breast cancer as second cancer, and the excess was more apparent for hematopoietic diseases such as myeloid leukemia (5,6). Acute non-lymphocytic leukemia (ANLL), including acute myeloid leukemia (AML), is the predominant subsequent hematopoietic disease among breast cancer survivors. The second ANLL developed by breast cancer survivors is possibly associated with the side effects of adjuvant therapy or etiological associations with various forms of malignancy. Moreover, several studies reported that breast cancer patients treated with chemotherapy and/or radiation therapy had an increased risk of developing AML, which was called therapy-related acute myeloid leukemia (t-AML) (7).
Here, we systemically assessed subsequent primary leukemia incidence among a large cohort of subjects with breast cancer, and we identified risk factors and outcomes for subsequent primary ANLL among breast cancer patients. We calculated the standardized incidence ratio (SIR) for subsequent primary cancers following breast cancer based on the Surveillance, Epidemiology, and End Results (SEER) database. Multivariate analysis was conducted to investigate the risk factors behind developing a second primary ANLL following breast cancer and to assess survival outcomes.
Methods
Data sources and study design
Data were collected from the SEER 18 Registries database, which brings together cancer survival, incidence, and tumor characteristics information from a number of geographical areas across the United States.
From this database, female patients with breast cancer alone and patients who had survived breast cancer but went on to develop second leukemia who received their diagnosis between January 1, 2000, and December 31, 2014, were selected. Patients who received their second primary cancer diagnosis in the 6 months after initially being diagnosed with breast cancer were not included as these had a high possibility of being pre-existing or synchronous cancers (8). The follow-up period lasted up to the date any second cancer was diagnosed, death occurred due to any cause, the date of last known vital status, or when the study concluded (December 31, 2014). None of the follow-up data were excluded from the Cox proportional hazards regression model analyses of risk factors and outcomes for the subsequent development of ANLL after breast cancer.
We found data of 617,083 women who had breast cancer along and 1,555 patients who developed subsequent leukemia after being diagnosed with primary breast cancer. Overall, 926 individuals who developed a second ANLL, and 919 patients with follow-up data were included in the analyses of risk factors and outcomes. Furthermore, we performed analyses on the characteristics of the first breast cancer, including the year of diagnosis (2000–2004, 2005–2009, and 2010–2014), age at diagnosis (aged under 14, 15–39, and 40+ years) (9), latency period (6–11, 12–59, 60–119, and 120+ months), subtype (ER+PR+, ER+PR−, ER−PR+, or ER−PR−), radiation (none or yes), and chemotherapy (none or yes).
Statistical analysis
Estimating the SIR
The SEER*Stat Multiple primary-standardized incidence ratios (MP-SIR) tool (version 8.3.4) was employed to draw comparisons with the relative risk in the population generally. The tool enables the SIRs to be calculated by dividing the observed numbers of second primary cancers by the anticipated numbers of second primary cancers based on the rates seen in the general population, along with the 95% confidence interval (95% CI). CIs and P values were at 0.05 significance alpha levels and were two-sided based on Poisson exact methods. In cases where the number of observed cases was zero, the SIRs and CIs of the 0–14 age group were not put forward, with the intention of avoiding estimations with statistical instability (10).
Risk factor analysis
Chi-square tests were carried out to compare the characteristics of the women in both groups (breast cancer alone and second primary ANLL). Evaluations of the hazard ratios (HRs) and corresponding 95% CIs were performed by crude semi-parametric Cox proportional hazards regression analyses, which also showed the risk factors behind developing second primary ANLL following breast cancer. The latency period was commenced at the initial diagnosis of breast cancer diagnosis and censored at the diagnosis of second cancer.
Survival outcomes
To allow for adjustment for other prognostic indicators, the association with survival was analyzed by multivariable Cox regression. Overall survival (OS) was the main endpoint. OS was defined as the period from surgery up to the date of death (due to any cause) or the date of the last follow-up. SPSS 19.0 (IBM Corporation, Armonk, NY, USA) generated all statistical analyses and charts. P<0.05 signified statistical significance. Every test conducted was two-sided.
Results
SIRs for ANLL among breast cancer survivors
A total of 1,555 patients developed leukemia after the breast cancer diagnosis, including 926 women who suffered from ANLL, which was comprised predominately of AML and a small amount of acute monocytic leukemia (AMoL). The SIRs for leukemia are shown in Table S1. Increased SIRs were observed in breast cancer survivors with second ANLL (SIR: 2.41; 95% CI: 2.26–2.58), including second AML, AMoL, and other (SIR: 2.45; 95% CI: 2.28–2.62, SIR: 3.34; 95% CI: 2.55–4.30, and SIR: 1.30; 95% CI: 0.89–1.84, respectively).
Table 1 shows the SIRs for second primary ANLL among breast cancer survivors. A total of 926-second primary ANLL were examined and analyzed in our study. Across every year of breast cancer diagnosis, ANLL patients had increased SIRs, although this declined in more recent years (2000–2004 SIR: 2.90; 2005–2009 SIR: 2.59; 2010–2014 SIR: 2.24). Meanwhile, elevated SIRs were observed in the adolescent and young adult group (15–39 years, SIR: 18.12; 95% CI: 12.23–25.87), and the SIRs decreased with increasing age, as seen in the older adult group (40+ years, SIR: 2.35; 95% CI: 2.20–2.51). The SIRs were elevated for each latency period excluding the first 6–11 months post-diagnosis, and these decreased according to the time period (12–59 SIR: 3.23; 60–119 SIR: 1.86; 120+ SIR: 1.74). Overall, patients with 12–59 months’ latency had the highest SIR for developing ANLL. In addition, patients with all breast cancer subtypes had elevated SIRs for ANLL (SIR: ER+PR+ 2.25, ER+PR− 2.06, ER−PR+ 3.27, ER−PR− 3.98). Patients who underwent chemotherapy/radiation showed higher SIR for ANLL than those who did not receive chemotherapy/radiation. Meanwhile, breast cancer patients who were treated with combined chemotherapy and radiation had the highest risk for subsequent ANLL in comparison with the population generally (SIR: 5.75; 95% CI: 5.17–6.38).
Table 1
| Characteristics | Acute non-lymphocytic leukemia | |
|---|---|---|
| O | SIR (95% CI) | |
| Calendar year of breast cancer diagnosis | ||
| 2000–2004 | 114 | 2.90*# (2.40−3.49) |
| 2005–2009 | 308 | 2.59*# (2.31−2.90) |
| 2010–2014 | 504 | 2.24*# (2.05−2.44) |
| Attained age, years | ||
| 15–39 | 30 | 18.12*# (12.23−25.87) |
| 40+ | 896 | 2.35*# (2.20−2.51) |
| Latency period, months | ||
| 6–11 | 33 | 1.21 (0.83−1.70) |
| 12–59 | 571 | 3.23*# (2.97−3.50) |
| 60–119 | 244 | 1.86*# (1.63−2.10) |
| 120+ | 75 | 1.74*# (1.37−2.18) |
| Unknown | 3 | 0.58 (0.12−1.17) |
| Subtype | ||
| ER+PR+ | 517 | 2.25*# (2.06−2.45) |
| ER+PR− | 91 | 2.06*# (1.66−2.53) |
| ER−PR+ | 11 | 3.27*# (1.63−5.84) |
| ER−PR− | 199 | 3.98*# (3.45−4.58) |
| Unknown | 108 | 1.77*# (1.39, 2.21) |
| Radiation | ||
| None | 354 | 2.00*# (1.80−2.22) |
| Yes | 524 | 2.91*# (2.67−3.17) |
| Unknown | 48 | 2.95*# (1.80−4.56) |
| Chemotherapy | ||
| None | 383 | 1.42*# (1.28−1.57) |
| Yes | 543 | 4.98*# (4.57−5.42) |
| Adjuvant treatment | ||
| R + chemotherapy | 356 | 5.75*# (5.17−6.38) |
| R only | 168 | 1.42*# (1.21−1.65) |
| Chemotherapy only | 158 | 3.84*# (3.27−4.49) |
| None | 196 | 1.44*# (1.25−1.66) |
| Unknown | 48 | 2.95*# (1.80−4.56) |
#, these values indicate the SIR (risk) for developing a second primary cancer was significantly increased. *P<0.05; confidence intervals are 95%. O, observed numbers; SIR, standardized incidence ratio; HR, hormone receptor; ER, estrogen receptor; PR, progesterone receptor; R, radiotherapy.
Risk factors for developing ANLL after breast cancer
Table 2 shows the clinical characteristics of the female patients who had breast cancer alone, together with those of the women who developed second primary ANLL. Women with subsequent ANLL differed significantly from patients with breast cancer only in terms of the race, grade, tumor size, ER and PR status, radiation, and chemotherapy, according to Chi-square tests (Table 2). In relation to age at diagnosis, no statistically significant differences were observed between women who had breast cancer alone and those with second primary ANLL.
Table 2
| Variables | Women with breast cancer only, N=617,083 (%) | Women with second acute non-lymphocytic leukemia, N=919 (%) | P value* |
|---|---|---|---|
| Age at diagnosis, y | 0.550 | ||
| ≤14 years | 11 (0.0) | 0 (0.0) | |
| 15–39 years | 36,796 (6.0) | 47 (5.1) | |
| 40+ years | 580,276 (94.0) | 872 (94.9) | |
| Race | 0.028# | ||
| White | 495,712 (80.3) | 758 (82.5) | |
| Black | 66,054 (10.7) | 97 (10.5) | |
| Other | 50,761 (8.2) | 64 (7.0) | |
| Unknown | 4,556 (0.8) | 0 (0.0) | |
| Grade | <0.001# | ||
| Well | 118,814 (19.3) | 154 (16.8) | |
| Moderately | 236,676 (38.4) | 341 (37.1) | |
| Poorly | 199,579 (32.3) | 349 (38.0) | |
| Undifferentiated | 6,632 (1.0) | 18 (2.0) | |
| Unknown | 55,382 (9.0) | 57 (6.1) | |
| Tumor size (mm) | <0.001# | ||
| ≤20 | 256,856 (41.6) | 263 (28.6) | |
| 21–50 | 142,188 (23.0) | 202 (22.0) | |
| 50+ | 35,293 (5.7) | 55 (6.0) | |
| Unknown | 182,746 (29.7) | 399 (43.4) | |
| ER | 0.007# | ||
| Negative | 114,117 (18.5) | 210 (22.8) | |
| Positive | 445,788 (72.3) | 630 (68.6) | |
| Borderline | 852 (0.1) | 2 (0.2) | |
| Unknown | 56,326 (9.1) | 77 (8.4) | |
| PR | <0.001# | ||
| Negative | 174,424 (28.3) | 289 (31.4) | |
| Positive | 377,994 (61.3) | 528 (57.5) | |
| Borderline | 2,615 (0.4) | 11 (1.2) | |
| Unknown | 62,050 (10.0) | 91 (9.9) | |
| Radiation | <0.001# | ||
| None | 315,573 (51.1) | 376 (40.9) | |
| Yes | 301,510 (48.9) | 543 (59.1) | |
| Chemotherapy | <0.001# | ||
| None/unknown | 361,386 (58.6) | 382 (41.6) | |
| Yes | 255,697 (41.4) | 537 (58.4) |
*, P values calculated by Pearson Chi squared testing; #, if statistically significant, P<0.05.
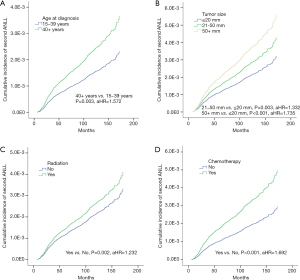
We then examined the risk factors of developing subsequent ANLL after breast cancer (Table 3) and found the risk of developing subsequent ANLL to be positively associated with age at breast cancer diagnosis (40+ vs. 15–39 years, aHR =1.572, P=0.003, Figure 1A), and tumor size (21–50 vs. ≤ 20 mm, aHR =1.332, P=0.003; 50+ vs. ≤ 20 mm, aHR =1.735, P<0.001; Figure 1B). Moreover, our study also suggested that the risk of developing ANLL among breast cancer patients was positively associated with adjuvant treatment. Compared with survivors who did not receive radiation treatment, women who were treated with radiation had an increased risk of developing ANLL (yes vs. no, aHR =1.232, P=0.002; Figure 1C). Similarly, patients who received chemotherapy had a higher risk of subsequent ANLL compared to those who did not receive chemotherapy (yes vs. no, aHR =1.692, P<0.001; Figure 1D). However, Table 3 showed that race, grade, and ER and PR status had no association with the risk.
Table 3
| Variables | Acute non-lymphocytic leukemia | |
|---|---|---|
| aHR (95% CI) | P value* | |
| Age at diagnosis, y | ||
| 15–39 years | Reference | |
| 40+ years | 1.572 (1.168−2.115) | 0.003# |
| Race | ||
| White | Reference | |
| Black | 1.023 (0.826−1.266) | 0.837 |
| Other | 0.842 (0.652−1.087) | 0.188 |
| Grade | ||
| Well | Reference | |
| Moderately | 1.008 (0.830−1.223) | 0.938 |
| Poorly | 1.102 (0.890−1.364) | 0.371 |
| Undifferentiated | 1.458 (0.887−2.399) | 0.137 |
| Tumor size (mm) | ||
| ≤20 | Reference | |
| 21–50 | 1.332 (1.101−1.611) | 0.003# |
| 50+ | 1.735 (1.289−2.334) | <0.001# |
| ER | ||
| Negative | Reference | |
| Positive | 0.865 (0.688−1.086) | 0.211 |
| PR | ||
| Negative | Reference | |
| Positive | 1.037 (0.846−1.270) | 0.728 |
| Radiation | ||
| None | Reference | |
| Yes | 1.232 (1.077−1.409) | 0.002# |
| Chemotherapy | ||
| None/unknown | Reference | |
| Yes | 1.692 (1.463−1.958) | <0.001# |
*, P values calculated by Log-rank testing; #, if statistically significant, P<0.05.
Outcomes following ANLL development
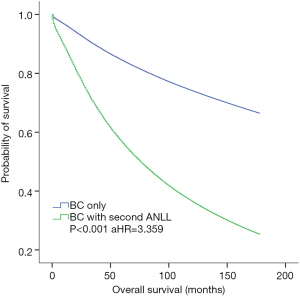
OS was defined as the period of time from breast cancer diagnosis to death due to any cause, including breast cancer-specific death or disease progression to ANLL or other related complications. Patients with breast cancer alone and those with breast cancer who developed subsequent ANLL were analyzed in our study. Table S2 shows that the multivariable Cox regression model was analyzed to adjust for other prognostic indicators of OS. Breast cancer survivors who developed ANLL had a significantly worse OS compared with patients who did not develop ANLL (aHR =3.359, P<0.001; Figure 2). The survival of breast cancer patients with second ANLL was comparable between those who received chemotherapy/radiation and those who did not receive chemotherapy/radiation (both P>0.05, not present).
Discussion
ANLL is one of the most common subsequent myeloid neoplasms (MNs) encountered by breast cancer survivors, and it comprises a predominant number of AML and a small amount of acute monocytic leukemia (AMoL). In this study, the comprehensive, retrospective-based analysis of a large population revealed dynamic changes in the risk of subsequent ANLL with first primary breast cancer. The results observed indicated that patients with breast cancer had an increased risk of developing ANLL compared to the general population. In particular, we found that adults with lower age, a short latency period (1–5 years), and ER-PR- subtype had higher SIR for subsequent ANLL, and patients with adjuvant treatment including chemotherapy and radiation also had significantly elevated SIR compared to the general population. Meanwhile, the risk of subsequent ANLL was positively associated with age, tumor size, chemotherapy, and radiation in women with first breast cancer. In addition, a truncated OS was observed in breast cancer patients who developed ANLL, which was adjusted for other independent prognostic factors.
Our results showed that, in correlation with previous studies, breast cancer survivors have an increased risk of developing ANLL compared with the population generally (5,6). Our study also unveiled the trend, which shows the decline in SIR of subsequent ANLL in more recent years. However, this trend could be a mere reflection of the shortened latency period for breast cancer survivors witnessed over the same period. Previous studies observed the highest absolute excess risk of second primary cancers in adolescents and young adult survivors (AYAs), compared with all age groups (11). In addition, another study demonstrated that young women with breast cancer had the highest risk of developing therapy-related myeloid neoplasm (t-MN) (12), our study also suggested AYAs had significantly higher SIR of subsequent ANLL compared to older adult patients after the first breast cancer. There was an age-dependent SIR of subsequent ANLL in AYAs and older adult survivors, which could be potentially be attributed to young females with early on-set breast cancer having a germline mutation in BRAC1 or BRAC2, which leads to enhanced susceptibility (13,14). Although subsequent ANLL is less common among AYAs with breast cancer, the fact that AYAs had such a high SIR for developing ANLL is of clinical importance (15). As found in prior studies, subsequent leukemia may not present within 1 year and may occur at a few latency period (16). Meanwhile, ER-PR- breast cancer patients had the highest SIR of the second ANLL, which could be explained by different genetic predisposition among different subtypes, and greater chemotherapy exposure for breast cancer in ER-PR- patients, according to more probable aggressive biologic behaviors (17).
Adjuvant therapy, such as chemotherapy and radiation, plays a pivotal role in the management of breast cancer patients, with the adverse impact of therapy-related complications considered in recent years. As is known, t-MN is a typical complication of chemotherapy and radiation for breast cancer (18), and therapy-related acute non-lymphocytic leukemia (t-ANLL) is believed to be the consequence of metabolism alteration and genomic changes induced by therapy (19,20). The tumor type related to chemotherapy is mainly t-AML in patients with t-ANLL, with exposure to cyclophosphamide and epirubicin, but not to taxanes (21,22). Despite this, we did not carry out the evaluation of the impact of different doses and regimens of chemotherapy as dose and regimen information to is not available from the SEER database. Prior observations reported that radiation therapy has a stronger relation to acute promyelocytic leukemia (APL) than to other ANLL types, and the latency period of radiation-associated ANLL can vary (23). However, the risk of t-MN after exposure to radiation (external beam radiation) is probably lower in comparison with chemotherapy or combined chemotherapy and radiation, as shown by our study (24).
We had extensive information on risk factors and survival outcomes for developing subsequent ANLL among breast cancer survivors. For breast cancer survivors, in the development of subsequent ANLL, older age (40+ years), larger tumor size, chemotherapy, and radiation treatment were in the main related to a heightened risk of subsequent ANLL. Meanwhile, this study corresponds well with previous findings that the development of subsequent MNs among survivors of first unrelated primary cancer can increase mortality and worsen OS (9).
Our study had both strengths and limitations. Contributing to its reliability was having data from a large, well-established and standardized population as its foundation. However, it was also a retrospective study, focusing on a heterogeneous population from the SEER data collection. Furthermore, as breast cancer survivors are likely to be given more attention in relation to potential complications than the population as a whole, it is plausible that a surveillance bias might exist for survivors. Therefore, we focused on a 15-year period and conducted an analysis of data from 18 registries, with these systemic errors non-differential. Another limitation was potentially pre-existing or synchronous cancers. To address these issues, this study excluded patients with subsequent leukemia diagnosed within 6 months of a breast cancer diagnosis. Moreover, there is a lack of detailed adjuvant therapy information and genetic information. Lastly, due to the composition of the dataset, the numbers of AYAs for subsequent ANLL among breast cancer survivors were small. Our findings, therefore, require further confirmation.
In conclusion, we found breast cancer survivors to have a higher risk of developing subsequent ANLL compared to the population as a whole. The heightened risk of developing subsequent ANLL could possibly be related to the biological behavior of the tumor, therapy exposure, and gene susceptibility. In addition, age, tumor size, chemotherapy, and radiation are risk factors for the development of subsequent ANLL among breast cancer survivors. Older age, larger tumor size, chemotherapy, and radiation in survivors are positively associated with a higher risk of subsequent ANLL. Meanwhile, survivors following a subsequent ANLL had an adverse survival outcome. These findings are useful for health planning, including monitoring and the development of specific guidelines for both the general population and breast cancer patients.
Table S1
| Sites | O | SIR (95% CI) |
|---|---|---|
| Leukemia | 1,555 | 1.43*# (1.36−1.51) |
| Lymphocytic leukemia | 414 | 0.80*& (0.73−0.88) |
| Acute lymphocytic leukemia | 84 | 1.95*# (1.56−2.42) |
| Chronic lymphocytic leukemia | 318 | 0.70*& (0.63−0.79) |
| Other lymphocytic leukemia | 12 | 0.55*& (0.29−0.97) |
| Non-lymphocytic leukemia | 1,141 | 2.01*# (1.89−2.13) |
| Acute non-lymphocytic leukemia | 926 | 2.41*# (2.26−2.58) |
| Acute myeloid leukemia | 834 | 2.45*# (2.28−2.62) |
| Acute monocytic leukemia | 60 | 3.34*# (2.55−4.30) |
| Other | 32 | 1.30 (0.89−1.84) |
| Chronic non-lymphocytic leukemia | 167 | 1.25*# (1.07−1.45) |
| Other | 48 | 1.66*# (1.03−2.54) |
#, these values indicate the SIR (risk) for developing a second primary cancer was significantly increased; &, these values indicate the SIR (risk) for developing a second primary cancer was significantly decreased; *P<0.05; confidence intervals are 95%. O, observed numbers; SIR, standardized incidence ratio; NHL, non-Hodgkin lymphoma.
Table S2
| Variables | Acute non-lymphocytic leukemia | |
|---|---|---|
| aHR (95% CI) | P value | |
| Patients | ||
| Breast cancer only | Reference | |
| With second ANLL | 3.359 (3.123−3.614) | <0.001# |
| Age at diagnosis, y | ||
| 15–39 years | Reference | |
| 40+ years | 1.497 (1.460−1.534) | <0.001# |
| Race | ||
| White | Reference | |
| Black | 1.303 (1.283−1.324) | <0.001# |
| Other | 0.672 (0.656, 0.688) | <0.001# |
| Grade | ||
| Well | Reference | |
| Moderately | 1.346 (1.322−1.371) | <0.001# |
| Poorly | 1.795 (1.761−1.830) | <0.001# |
| Undifferentiated | 2.399 (2.349−2.450) | <0.001# |
| Tumor size (mm) | ||
| ≤20 | Reference | |
| 21–50 | 2.241 (2.202−2.281) | <0.001# |
| 50+ | 4.928 (4.821−5.038) | <0.001# |
| ER | ||
| Negative | Reference | |
| Positive | 0.914 (0.898−0.931) | <0.001# |
| PR | ||
| Negative | Reference | |
| Positive | 0.756 (0.744−0.768) | <0.001# |
| Radiation | ||
| None | Reference | |
| Yes | 0.595 (0.588−0.602) | <0.001# |
| Chemotherapy | ||
| None/unknown | Reference | |
| Yes | 0.674 (0.666−0.683) | <0.001# |
*, P values calculated by Log-rank testing; #, if statistically significant, P<0.05.
Acknowledgments
Funding: This work was partially supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.62). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 2016;66:31-42. [Crossref] [PubMed]
- Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 2016;122:3075-86. [Crossref] [PubMed]
- Jabagi MJ, Vey N, Goncalves A, et al. Evaluation of the Incidence of Hematologic Malignant Neoplasms Among Breast Cancer Survivors in France. JAMA Netw Open 2019;2:e187147. [Crossref] [PubMed]
- Bazire L, De Rycke Y, Asselain B, et al. Risks of second malignancies after breast cancer treatment: Long-term results. Cancer Radiother 2017;21:10-5. [Crossref] [PubMed]
- Ricceri F, Fasanelli F, Giraudo MT, et al. Risk of second primary malignancies in women with breast cancer: Results from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 2015;137:940-8. [Crossref] [PubMed]
- Wei JL, Jiang YZ, Shao ZM. Survival and chemotherapy-related risk of second primary malignancy in breast cancer patients: a SEER-based study. Int J Clin Oncol 2019;24:934-40. [Crossref] [PubMed]
- McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer 2017;17:513-27. [Crossref] [PubMed]
- Liu J, Jiang W, Mao K, et al. Elevated risks of subsequent endometrial cancer development among breast cancer survivors with different hormone receptor status: a SEER analysis. Breast Cancer Res Treat 2015;150:439-45. [Crossref] [PubMed]
- Keegan THM, Bleyer A, Rosenberg AS, et al. Second Primary Malignant Neoplasms and Survival in Adolescent and Young Adult Cancer Survivors. JAMA Oncol 2017;3:1554-7. [Crossref] [PubMed]
- Kim C, Bi X, Pan D, et al. The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid 2013;23:575-82. [Crossref] [PubMed]
- Lee JS, DuBois SG, Coccia PF, et al. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer 2016;122:116-23. [Crossref] [PubMed]
- Martin MG, Welch JS, Luo J, et al. Therapy related acute myeloid leukemia in breast cancer survivors, a population-based study. Breast Cancer Res Treat 2009;118:593-8. [Crossref] [PubMed]
- Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol 2008;26:4282-8. [Crossref] [PubMed]
- Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol 2018;19:169-80. [Crossref] [PubMed]
- Hulegårdh E, Nilsson C, Lazarevic V, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol 2015;90:208-14. [Crossref] [PubMed]
- Chaplain G, Milan C, Sgro C, et al. Increased risk of acute leukemia after adjuvant chemotherapy for breast cancer: a population-based study. J Clin Oncol 2000;18:2836-42. [Crossref] [PubMed]
- Sun J, Meng H, Yao L, et al. Germline Mutations in Cancer Susceptibility Genes in a Large Series of Unselected Breast Cancer Patients. Clin Cancer Res 2017;23:6113-9. [Crossref] [PubMed]
- Guru Murthy GS, Abedin S. Myeloid malignancies after treatment for solid tumours. Best Pract Res Clin Haematol 2019;32:40-6. [Crossref] [PubMed]
- Churpek JE, Marquez R, Neistadt B, et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer 2016;122:304-11. [Crossref] [PubMed]
- Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015;518:552-5. [Crossref] [PubMed]
- Matesich SM, Shapiro CL. Second cancers after breast cancer treatment. Semin Oncol 2003;30:740-8. [Crossref] [PubMed]
- Sevcikova K, Zhuang Z, Garcia-Manero G, et al. Comprehensive analysis of factors impacting risks and outcomes of therapy-related myeloid neoplasms following breast cancer treatment. Leukemia 2016;30:242-7. [Crossref] [PubMed]
- Radivoyevitch T, Sachs RK, Gale RP, et al. Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation. Leukemia 2016;30:285-94. [Crossref] [PubMed]
- Nardi V, Winkfield KM, Ok CY, et al. Acute myeloid leukemia and myelodysplastic syndromes after radiation therapy are similar to de novo disease and differ from other therapy-related myeloid neoplasms. J Clin Oncol 2012;30:2340-7. [Crossref] [PubMed]


