
High programmed death-ligand 1 expression is a poor prognostic indicator for esophageal squamous cell carcinoma and is correlated with two-field lymph node metastasis
Introduction
Esophageal cancer is the seventh most malignant tumors; with high mortality, it also ranks sixth in cancer-related deaths worldwide (1). The two major histological types are esophageal adenocarcinoma (EC) and esophageal squamous cell carcinoma (ESCC). The former frequently occurs in western countries, while the latter is more predominant in Asian countries, particularly China, accounting for more than 90% of the total cases (2). Despite advances in therapy such as surgery, radiation therapy, chemotherapy, and biological therapy, the prognosis of ESCC patients remains poor, with a 5-year overall survival rate of 20–30% (3). Among related factors, lymph node metastasis (LNM) is central to unfavorable prognosis. Therefore, there is a need to identify effective prognostic biomarkers, to develop innovative therapeutics, and to improve ESCC patient survival.
The programmed cell death 1/programmed death-ligand 1 (PD-1/PD-L1) signaling pathway plays a vital role in tumor immune escape (4), leading to the poor prognosis of cancer patients (5). Several studies have demonstrated the association of PD-L1 expression with adverse outcomes of patients with various types of malignant tumors, including non-small cell lung cancer, gastric cancer, renal cell carcinoma, colorectal cancer, and breast cancer (6-9). Immunological checkpoint inhibitors targeting the PD-1/PD-L1 pathway in a variety of human tumor therapies are promising therapeutics (10). However, as only a minority of patients can benefit from immunotherapy, the identification of predictive indicators to guide treatment decisions is of widespread concern in clinical practice. Although the prognostic value of PD-L1 expression in ESCC patients has been partly investigated, the conclusions remain controversial. Therefore, the present study further assessed PD-L1 expression in ESCC to determine its correlation with clinicopathological features and prognosis and explore the association between PD-L1 expression and LNM status in ESCC to provide a reference for the clinical treatment of esophageal cancer.
Methods
Patients and samples
The present study enrolled 108 patients with ESCC who underwent minimally invasive esophagectomy in the Thoracic Department of Fujian Medical University Union Hospital between January 2012 and March 2014. No patients received preoperative neoadjuvant therapy. The clinicopathologic features of each patient are summarized in Table 1. The clinical stages of the tumors were determined according to the 8th American Joint Commission on Cancer TNM staging system. Complete follow-up data from the postoperative period were available for these patients. This study was approved by the ethics committee of Fujian Medical University Union Hospital, Fuzhou, China (No. 2016KY012).
Table 1
| Index | Cases (n) | Percent (%) |
|---|---|---|
| Age (year) | ||
| <60 | 61 | 56.5 |
| ≥60 | 47 | 43.5 |
| Gender | ||
| Female | 29 | 26.9 |
| Male | 79 | 73.1 |
| Nerve invasion | ||
| No | 88 | 81.5 |
| Yes | 20 | 18.5 |
| Vascular invasion | ||
| No | 91 | 84.3 |
| Yes | 17 | 15.7 |
| Tumor size (cm) | ||
| <4 | 91 | 84.3 |
| ≥4 | 17 | 15.7 |
| Tumor grading | ||
| G1 | 36 | 33.3 |
| G2 | 61 | 56.5 |
| G3 | 11 | 10.2 |
| Tumor location | ||
| Upper | 7 | 6.5 |
| Middle | 68 | 62.9 |
| Low | 33 | 30.6 |
| T stage | ||
| T1a | 21 | 19.4 |
| T1b | 15 | 13.9 |
| T2 | 56 | 51.9 |
| T3 | 16 | 14.8 |
| N stage | ||
| N0 | 62 | 57.4 |
| N1 | 31 | 28.7 |
| N2 | 11 | 10.2 |
| N3 | 4 | 3.7 |
| TNM stage | ||
| I | 18 | 16.7 |
| II | 40 | 37 |
| III | 43 | 39.8 |
| IV | 7 | 6.5 |
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue samples were serially cut into 4-µm-thick sections for immunohistochemical (IHC) analysis. In brief, sections were deparaffinized and hydrated, followed by antigen retrieval (95 °C, 20 min). After blocking endogenous peroxidase activity, the sections were incubated with anti-PD-L1 rabbit monoclonal primary antibody (dilution 1:50; ab213524; Abcam, Cambridge, MA, USA) for 2 hours at room temperature or overnight at 4 °C. Following three rinses in phosphate-buffered saline (PBS), the sections were incubated with an anti-rabbit polymer (Envision Plus; Fuzhou Maixin Biotechnology Company, Fuzhou, China) for 15 min at room temperature. Thereafter, staining was performed with a 3,3-diaminobenzidine (DAB) solution. The sections were visualized with DAB and lightly counterstained with hematoxylin for histological observation on a light microscope (11).
Evaluation of immunostaining
All slides were evaluated independently by two senior pathologists who were blinded to patients’ clinicopathological characteristics. The proportion of stained tumor cells was semi-quantitatively scored as 0% (n=0), 1–30% (n=1), 30–60% (n=2), and 60–100% (n=3), while the predominant PD-L1 staining intensity was scored as negative (n=0), weak (n=1), moderate (n=2), or strong (n=3) (12). PD-L1 expression on the normal tonsil tissues was used as a positive control. The final score of each patient was calculated by multiplying the score for the proportion of stained tumor cells by the intensity score (ranging from 0 to 9). Samples with scores ≥4 were classified as high expression, while the remaining samples were considered low expression.
Statistical analysis
The statistical analyses were conducted using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). The clinicopathological factors were compared between groups by Pearson’s chi-square or Fisher’s exact tests. The Kaplan-Meier method and log-rank tests were used for survival curve analysis and to determine statistical differences in GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA). Univariate and multivariate survival analysis adopted a Cox proportional hazards regression model. P values <0.05 in all statistical tests were statistically significant.
Results
Patient characteristics
As shown in Table 1, the cohort comprised 29 (26.9%) female and 79 (73.1%) male patients with a median age of 60 years (range, 31–71 years). Seventeen (15.7%) and 20 (18.5%) patients had vascular and nerve invasion, respectively. Seven (6.5%), 68 (62.9%), and 33 (30.6%) tumors were in the upper, middle, and lower locations, respectively. Most patients (84.3%) had tumors <4 cm in diameter. The tumor grades included low (36 patients, 33.3%), intermediate (61, 56.5%) and high (11, 10.2%). By staging, 21 (19.4%), 15 (13.9%), 56 (51.9%), and 16 (14.8%) tumor were T1a, T1b, T2, and T3, respectively. Regarding LNM, 46 (42.6%) patients were node-positive (28.7% in N1, 10.2% in N2 and 3.7% in N3). Among all patients, 18 (16.7%), 40 (37.0%), 43 (39.8%), and 7 (6.5%) were classified as stage I, II, III, and IV, respectively according to TNM staging.
Association of PD-L1 expression with clinicopathological characteristics of patients with ESCC
PD-L1 protein expression was examined by IHC in 108 ESCC tissue samples. Representative images of negative expression and weak, moderate, and strong PD-L1 staining are shown in Figure 1. PD-L1 IHC staining was predominantly present on the cell membrane of ESCC tissues. Stained tonsil tissue was used as a positive control. PD-L1 expression in the ESCC specimens in the present study was categorized as low (<4) and high (≥4) for further analysis according to the evaluation scores defined above. In this study, 43.5% of cases showed high PD-L1 expression, while 56.5% showed low expression. Analysis of the associations between clinicopathological characteristics and PD-L1 expression showed that only N stage was significantly correlated with high expression (P=0.038), while age (P=0.859), sex (P=0.113), nerve invasion (P=0.882), vascular invasion (P=0.832), tumor size (P=0.788), tumor grade (P=0.071), tumor location (P=0.816), T stage (P=0.991), and TNM stage (P=0.070) were not (Table 2).
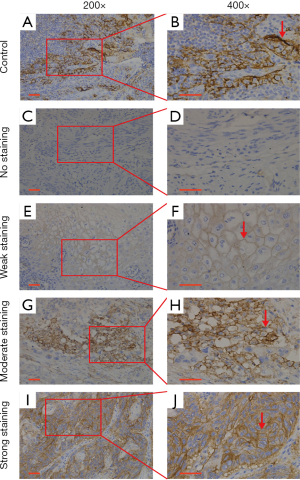
Table 2
| Variable | Cases | PD-L1 expression | χ2 score | P value | |
|---|---|---|---|---|---|
| Low (n=61) | High (n=47) | ||||
| Age (year) | 0.032 | 0.859 | |||
| <60 | 61 | 34 | 27 | ||
| ≥60 | 47 | 27 | 20 | ||
| Gender | 2.514 | 0.113 | |||
| Female | 29 | 20 | 9 | ||
| Male | 79 | 41 | 38 | ||
| Nerve invasion | 0.022 | 0.882 | |||
| No | 88 | 50 | 38 | ||
| Yes | 20 | 11 | 9 | ||
| Vascular invasion | 0.045 | 0.832 | |||
| No | 91 | 51 | 40 | ||
| Yes | 17 | 10 | 7 | ||
| Tumor size (cm) | 0.072 | 0.788 | |||
| <4 | 75 | 43 | 32 | ||
| ≥4 | 33 | 18 | 15 | ||
| Tumor grading | 5.19 | 0.071 | |||
| G1 | 36 | 24 | 12 | ||
| G2 | 61 | 34 | 27 | ||
| G3 | 11 | 3 | 8 | ||
| Tumor Location | 0.637 | 0.816 | |||
| Upper | 7 | 3 | 4 | ||
| Middle | 68 | 39 | 29 | ||
| Low | 33 | 19 | 14 | ||
| T stage | 0.184 | 0.991 | |||
| T1a | 21 | 12 | 9 | ||
| T1b | 15 | 9 | 6 | ||
| T2 | 56 | 31 | 25 | ||
| T3 | 16 | 9 | 7 | ||
| N stage | 7.881 | 0.038* | |||
| N0 | 62 | 41 | 21 | ||
| N1 | 31 | 16 | 15 | ||
| N2 | 11 | 3 | 8 | ||
| N3 | 4 | 1 | 3 | ||
| TNM stage | 7.002 | 0.07 | |||
| I | 18 | 10 | 8 | ||
| II | 40 | 27 | 13 | ||
| III | 43 | 23 | 20 | ||
| IV | 7 | 1 | 6 | ||
*, indicates as statistical significance (P≤0.05).
Overall survival (OS) in ESCC
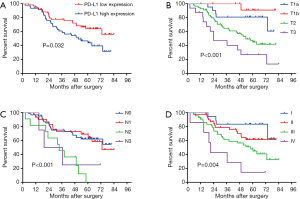
The median follow-up duration was 54.1 months (range, 2.3–84.1 months). Univariate and multivariate analyses were conducted to identify clinicopathological features affecting the prognosis of ESCC patients. As shown in Table 3, univariate Cox regression showed that T stage (P<0.001), N stage (P<0.001), TNM stage (P=0.004), and high PD-L1 expression (P=0.032) were prognostic factors contributing to poor 5-year OS (Table 3). Moreover, multivariate analysis revealed that T stage (P=0.001), N stage (P=0.031) and PD-L1 expression (P=0.049) were independent prognostic factors. In this study, Kaplan-Meier analyses demonstrated unfavorable OS in patients with high PD-L1 expression compared to that in patients with low PD-L1 expression (Figure 2A). Furthermore, T, N, and TNM stage were also significantly related to patient OS (Figure 2B,C,D).
Table 3
| Variable | Cases | univariate analysis | multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age(year) | ||||||||
| <60 | 61 | 1 | ||||||
| ≥60 | 47 | 1.661 | 0.942–2.929 | 0.080 | ||||
| Gender | ||||||||
| Female | 29 | 1 | ||||||
| Male | 79 | 0.803 | 0.429–1.502 | 0.492 | ||||
| Nerve invasion | ||||||||
| No | 88 | 1 | ||||||
| Yes | 20 | 1.773 | 0.936–3.358 | 0.079 | ||||
| Vascular invasion | ||||||||
| No | 91 | 1 | ||||||
| Yes | 17 | 1.821 | 0.927–3.574 | 0.082 | ||||
| Tumor size, cm | ||||||||
| <4 | 65 | 1 | ||||||
| ≥4 | 33 | 1.354 | 0.748–2.449 | 0.317 | ||||
| Tumor grading | ||||||||
| G1 | 36 | 1 | ||||||
| G2/3 | 72 | 1.201 | 0.652–2.215 | 0.557 | ||||
| Tumor location | ||||||||
| Upper/middle | 75 | 1 | ||||||
| Low | 33 | 0.630 | 0.327–1.214 | 0.167 | ||||
| T stage | ||||||||
| T1 | 36 | 1 | 1 | |||||
| T2–3 | 72 | 4.657 | 1.978–10.968 | <0.001* | 4.168 | 1.727–10.060 | 0.001* | |
| N stage | ||||||||
| N0-1 | 93 | 1 | 1 | |||||
| N2–3 | 15 | 3.565 | 1.857–6.843 | <0.001* | 2.129 | 1.073–4.224 | 0.031* | |
| TNM stage | ||||||||
| I–II | 58 | 1 | 1 | |||||
| III–IV | 50 | 2.385 | 1.328–4.282 | 0.004* | 0.904 | 0.426–1.920 | 0.794 | |
| PD-L1 expression | ||||||||
| Low | 61 | 1 | 1 | |||||
| High | 47 | 1.869 | 1.056–3.310 | 0.032* | 1.81 | 1.001–3.277 | 0.049* | |
*, indicates as statistical significance (P≤0.05). HR, hazard ratio; CI, confidence interval.
PD-L1 high expression correlated with LNM
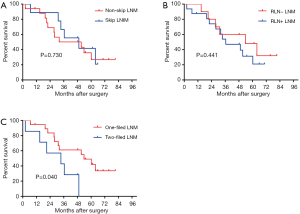
The subgroup analysis stratified patients with both high PD-L1 expression and LNM into three LNM statuses: skip- or skip+ LNM, the LNM around recurrent laryngeal nerve (RLN+ or RLN− LNM), and the different operative fields of LNM (two-field or one-field LNM). The results revealed that skip (P=0.730) and RLN (P=0.441) LNM status were not associated with a worse prognosis (Figure 3A,B), whereas the OS of two-field LNM (0%) was significantly lower than that of one-field LNM (49.0%) (P=0.040) (Figure 3C). In this study, skip LNM was defined as LNM out of the adjacent lymph node areas. For example, the adjacent lymph nodes for the middle thoracic ESCC were the upper, middle, and lower thoracic lymph nodes, while cervical and abdomen lymph nodes metastasis were considered skip LNM.
Discussion
Recent advances in cancer immunology have emphasized the importance of the PD-1 signaling pathway (13). PD-1 is a negative costimulatory receptor expressed mainly on activated T cells. The interaction of PD-1 with its ligands, PD-L1 or PD-L2, plays an important role in antigen-specific T-cell response, mediating PD-1-dependent immune suppression, which facilitates tumor cell escape from immune surveillance and promotes tumor progression (4,14,15). PD-L1 can be expressed in both tumor and immune cells (16). In this study, the PD-L1 was widely expressed in ESCC tissue samples, with high expression observed in 43.5% of samples. This result was consistent with those previously reported by Ohigashi et al. (43.9%) (17) and Chen et al. (41.4%) (18).
The relationship between PD-L1 expression and prognosis in esophageal cancer is controversial and difficult to clarify (19-22). A recent meta-analysis of 18 published studies and 3,306 patients with esophageal cancer reported summary statistics indicating that PD-L1 overexpression had an unfavorable impact on OS, with a pooled hazard ratio (HR) of 1.42 (95% CI: 1.09–1.86) (23). In the present study, both univariable and multivariable survival analyses demonstrated that high PD-L1 expression was an independent prognostic factor (HR: 1.810, 95% CI: 1.001–3.277, P=0.049), a finding consistent with previous reports. In addition, Kaplan-Meier survival analyses showed that ESCC patients with high PD-L1 expression had more unfavorable clinical outcomes compared to those in patients with low PD-L1 expression. Our results provided new evidence and reconfirmed that high PD-L1 expression predicts adverse clinical outcomes in patients with ESCC.
Increasing numbers of studies have semi-quantitatively detected PD-L 1 expression in tumor tissues by IHC. PD-L1 expression is heterogeneous, which may be related to the accuracy of IHC detection in different studies. The main factors are as follows: first, the antibody sensitivities and concentrations my not be the same. Secondly, the evaluation criteria for high PD-L1 expression varied in different studies (18). Finally, PD-L1 heterogeneity in tumors and different sampling times and sampled areas can also affect PD-L1 staining results (24).
The patients in the present study were classified into high and low PD-L1 expression groups. We observed a significant correlation between high PD-L1 expression and N stage, similar to a previous finding (17). LNM is widely regarded as the key factor affecting 5-year OS in ESCC patients; however, skip LNM, RLN LNM, and the operative fields LNM remain fiercely contested by scholars at home and abroad (25-27). The results of this study showed that PD-L1 expression and N stage were both independent prognostic factors in ESCC patients. Subgroup analyses were conducted to determine the effects of LNM status on prognosis in patients with high PD-L1 expression. The high PD-L1 expression group was further divided into three groups according to LNM status. We observed a lower 5-year OS rate in patients with two-field LNM than that in patients with one-field LNM, while skip and RLN LNM showed no prognostic significance.
The present study first explored the correlation between prognosis and skip LNM in ESCC patients. However, we observed no difference in 5-year OS between these two groups (52.0% in the skip+ and 36.9% in the skip− LNM groups, P=0.286). Moreover, lymph node dissection around the recurrent laryngeal nerve is the focus of esophagectomy (26). The harvested number and quality of lymph node dissection in this site directly affect the recurrence and long-term survival of ESCC patients (28). Therefore, this study analyzed the impact of RLN LNM on the prognosis of ESCC patients. We observed no significant difference in 5-year OS between RLN+ (54.2%) and RLN− (43.6%) LNM (P=0.387).
Did esophagectomy accompany two-field or three-field lymphadenectomy? Western scholars have suggested that LNM is a systemic disease, especially cervical LNM, which is not an option for radical dissection through surgery. However, eastern scholars, including those in China and Japan, consider cervical LNM to be regional metastasis; thus, they are a local disease and should undergo radical resection. These different views partially stem from the different esophageal pathology types between Western and Eastern countries (27,29,30). In this study, the 5-year OS of two-field LNM was only 12.1%, significantly lower than that of one-field LNM (58.6%, P=0.037). This finding has important implications for the development of individualized treatment strategies, suggesting the need for moderated expanded lymphadenectomy for ESCC patients with high PD-L1 expression. Expanded lymphadenectomy cannot only enhance the detection rate of LNM and facilitate accurate pathological staging but can also guide the comprehensive treatment and improve the OS of ESCC patients. Unfortunately, the present study did not include any patients with simultaneous three-field LNM, possibly due to the limited number of cases of this study and the fact that surgery was ruled out for patients with preoperative concurrent three-field LNM.
This study has several limitations. Firstly, the results might be influenced by selection bias due to the retrospective assessment of data from a single institution. Further validation is required in large-scale, multi-center prospective studies. Secondly, PD-L1 expression was evaluated from a partial tumor sample; thus, we could not differentiate tumor heterogeneity. Hence, the reproducibility of our results requires further validation. Finally, IHC was used to evaluate the clinical significance of PD-L1 but could not address mechanistic questions.
Conclusions
The results of this study demonstrated widespread PD-L1 expression in ESCC patients and that high PD-L1 expression was closely associated with N stage. High PD-L1 expression was also significantly related to poor 5-year OS and can serve as an independent prognostic biomarker for ESCC. In addition, our findings suggested that patients with simultaneous high PD-L1 expression and two-field LNM had a worse prognosis, providing further evidence of the significance of moderated expanded lymphadenectomy.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.22). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of Fujian Medical University Union Hospital, Fuzhou, China (No. 2016KY012) and written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Zheng B, Wu Z, Xue S, et al. hsa_circRNA_100873 upregulation is associated with increased lymphatic metastasis of esophageal squamous cell carcinoma. Oncol Lett 2019;18:6836-44. [PubMed]
- Straatman J, Joosten PJ, Terwee CB, et al. Systematic review of patient-reported outcome measures in the surgical treatment of patients with esophageal cancer. Dis Esophagus 2016;29:760-72. [Crossref] [PubMed]
- Chen L, Diao L, Yang Y, et al. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov 2018;8:1156-75. [Crossref] [PubMed]
- Jin Y, Zhao J, Shi X, et al. Prognostic value of programed death ligand 1 in patients with solid tumors: A meta-analysis. J Cancer Res Ther 2015;11:C38-43. [Crossref] [PubMed]
- Xia H, Shen J, Hu F, et al. PD-L1 over-expression is associated with a poor prognosis in Asian non-small cell lung cancer patients. Clin Chim Acta 2017;469:191-4. [Crossref] [PubMed]
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol 2019;46:3-10. [Crossref] [PubMed]
- Stenzel PJ, Schindeldecker M, Tagscherer KE, et al. Prognostic and Predictive Value of Tumor-infiltrating Leukocytes and of Immune Checkpoint Molecules PD1 and PDL1 in Clear Cell Renal Cell Carcinoma. Transl Oncol 2020;13:336-45. [Crossref] [PubMed]
- Zhang M, Sun H, Zhao S, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 2017;8:31347-54. [PubMed]
- Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J 2014;20:256-61. [Crossref] [PubMed]
- Zhou ZH, Ji CD, Zhu J, et al. The prognostic value and pathobiological significance of Glasgow microenvironment score in gastric cancer. J Cancer Res Clin Oncol 2017;143:883-94. [Crossref] [PubMed]
- Tanaka K, Miyata H, Sugimura K, et al. Negative influence of programmed death-1-ligands on the survival of esophageal cancer patients treated with chemotherapy. Cancer Sci 2016;107:726-33. [Crossref] [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207-12. [Crossref] [PubMed]
- Davis RJ, Ferris RL, Schmitt NC. Costimulatory and coinhibitory immune checkpoint receptors in head and neck cancer: unleashing immune responses through therapeutic combinations. Cancers Head Neck 2016;1:12. [Crossref] [PubMed]
- Niyongere S, Saltos A, Gray JE. Immunotherapy combination strategies (non-chemotherapy) in non-small cell lung cancer. J Thorac Dis 2018;10:S433-50. [Crossref] [PubMed]
- Romano E, Romero P. The therapeutic promise of disrupting the PD-1/PD-L1 immune checkpoint in cancer: unleashing the CD8 T cell mediated anti-tumor activity results in significant, unprecedented clinical efficacy in various solid tumors. J Immunother Cancer 2015;3:15. [Crossref] [PubMed]
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- Chen K, Cheng G, Zhang F, et al. Prognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinoma. Oncotarget 2016;7:30772-80. [PubMed]
- Momose K, Yamasaki M, Tanaka K, et al. MLH1 expression predicts the response to preoperative therapy and is associated with PD-L1 expression in esophageal cancer. Oncol Lett 2017;14:958-64. [Crossref] [PubMed]
- Hatogai K, Kitano S, Fujii S, et al. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget 2016;7:47252-64. [Crossref] [PubMed]
- Jesinghaus M, Steiger K, Slotta-Huspenina J, et al. Increased intraepithelial CD3+ T-lymphocytes and high PD-L1 expression on tumor cells are associated with a favorable prognosis in esophageal squamous cell carcinoma and allow prognostic immunogenic subgrouping. Oncotarget 2017;8:46756-68. [Crossref] [PubMed]
- Wakita A, Motoyama S, Nanjo H, et al. PD-L1 Expression Is a Prognostic Factor in Patients with Thoracic Esophageal Cancer Treated Without Adjuvant Chemotherapy. Anticancer Res 2017;37:1433-41. [Crossref] [PubMed]
- Yu W, Guo Y. Prognostic significance of programmed death ligand-1 immunohistochemical expression in esophageal cancer: A meta-analysis of the literature. Medicine (Baltimore) 2018;97:e11614. [Crossref] [PubMed]
- Wang X, Teng F, Kong L, et al. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016;9:5023-39. [Crossref] [PubMed]
- Wang Y, Zhu L, Xia W, et al. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag Res 2018;10:6295-303. [Crossref] [PubMed]
- Ma L, Xiang J, Zhang Y, et al. Characteristics and clinical significance of recurrent laryngeal nerve lymph node metastasis in esophageal squamous cell carcinoma. J BUON 2017;22:1533-9. [PubMed]
- Ma GW, Situ DR, Ma QL, et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol 2014;20:18022-30. [Crossref] [PubMed]
- Zheng W, Zhu Y, Guo CH, et al. Esophageal suspension method in scavenging peripheral lymph nodes of the left recurrent laryngeal nerve in thoracic esophageal carcinoma through semi-prone-position thoracoscopy. J Cancer Res Ther 2014;10:985-90. [Crossref] [PubMed]
- Shim YM, Kim HK, Kim K. Comparison of survival and recurrence pattern between two-field and three-field lymph node dissections for upper thoracic esophageal squamous cell carcinoma. J Thorac Oncol 2010;5:707-12. [Crossref] [PubMed]
- Shao L, Ye T, Ma L, et al. Three-field versus two-field lymph node dissection for thoracic esophageal squamous cell carcinoma: a propensity score-matched comparison. J Thorac Dis 2018;10:2924-32. [Crossref] [PubMed]


