
Extracellular ubiquitin inhibits the apoptosis of hepatoma cells via the involvement of macrophages
Introduction
Blood transfusion is a necessary treatment for cancer patients. Among multiple malignant cancers, the pathological characteristics of hepatocellular carcinoma (HCC), such as suppressed hematopoietic function, chronic blood loss, and intraoperative bleeding, blood transfusion is a vital treatment for HCC patients. However, several clinical and laboratory studies have confirmed the relationship between transfusion and the increased risk of recurrence and mortality in HCC (1-3). Storage time has been confirmed to influence the quality of red blood cells (RBCs), and the aging of RBCs in the storage bag (storage lesion) results in detrimental clinical outcomes, such as acute kidney/lung injury, pneumonia, infection, and cancer recurrence (4,5). Various biological molecules in stored RBCs are involved in transfusion-related immunomodulation (6) and may affect the recurrence and prognosis of HCC patients after the transfusion of aged RBCs.
Ubiquitin (Ub) is a highly conserved heat-stable 8.5 kDa protein in all eukaryotic cells, and the source of Ub may be the passive release from the necrotic and apoptotic cells (7). Lots of diseases can lead to increased Ub concentration in body fluids, such as type 2 diabetes, parasitic and allergic diseases, alcoholic liver disease, chronic hemodialysis, and traumatic injury (8). In addition, Ub can be released by normal cells (9,10). The concentration of Ub in RBC is 10–20 ng/106 cells and that is 1,000–10,000-fold higher than other blood cells. Ub in RBC units increased 20-fold on day 42 than day 0 due to the hemolysis occurred with the prolonged RBC storage (11,12). The most important effect of intracellular Ub is mediating protein degradation by the Ub-proteasome system. The protein degradation mediated by Ub exerts a vital influence on the regulation of multiple processes, such as protein trafficking, endocytosis, cell-cycle progression, transcriptional regulation, and signal transduction. The function of intracellular Ub is comparatively well studied, while less is known about the effect of extracellular ubiquitin (eUb) (13). In previous studies, eUb was shown to modulate the growth and apoptosis of hematopoietic cells (14), exert anti-inflammatory, and have an immunoregulation effect (15,16), which is supposedly related to the immunoregulatory properties of aged RBCs.
Due to the immunoregulation function of eUb and the development and progression of HCC depending on patients’ immunity, we speculated that the increased eUb during the storage of RBCs might play a vital role in the progression and recurrence of HCC after the transfusion of aged RBCs. However, there is a lack of research aimed at exploring the pro-tumor potential of eUb, so we intended to carry out an exploration into the impact eUb has on hepatoma cells and explore the potential mechanisms underlying HCC recurrence induced by the transfusion of aged RBCs, in the hope of alleviating the adverse outcomes of HCC patients after aged RBC transfusion treatment.
Methods
Cells and reagents
We purchased human hepatoma cell lines MHCC-97H and HepG2.2.15, human monocyte THP-1 cells from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). MHCC-97H and HepG2.2.15 were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (both Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), penicillin (100 U/mL) and streptomycin (100 µg/mL; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). THP-1 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. The incubation of the cells took place in a humidified incubator under 5% CO2 at 37 °C. eUb and PMA were sourced from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). We purchased all antibodies, including mTOR (#2983), p-mTOR (#2971), Akt (#4691), p-Akt (#9271), Bcl-xl (#2764), Bax (#5023), and GAPDH (#2118) from Cell Signaling Technology (Beverly, MA, USA).
CCK8 assay
Cells were seeded in 96-well plates (2×103 per well) and left to incubate for a period of 24 h. Each well had eUb added in a concentration series, and incubation was carried out for 24, 48, and 72 h, respectively. Further addition of 10 µL CCK8 was made to each well, and further incubation took place for 4 h at 37 °C. Absorbance was detected by a multi-mode detection platform (Molecular Devices Austria GmbH, Wals, Austria) at 490 nm.
Colony-formation assay
Different amounts of cells were seeded in 6-well plates: 100 cells/well in 97H and 200 cells/well in HepG2.2.15, respectively. The incubation of hepatoma cells was carried out with eUb over a period of 2 weeks, with the culture medium changed every 3 days. Then, we aspirated off the culture medium and washed the wells 3 times with PBS before fixing the cells with 20% methanol for 15 min. We stained the cells with Giemsa for 20 min and calculated the colony number to compare cell proliferation.
Cell apoptosis analysis
Flow cytometry was used to detect cell apoptosis via Annexin V-FITC/PI staining (KeyGen Biotech, Jiangsu, China). Then, 1×106 cells were washed with PBS and resuspended in 100 µL binding buffer containing 5 µL PI and 5 µL Annexin V-FITC. Incubating these cells at room temperature in the dark for 15 min according to the manufacturer’s instructions. Lastly, analysis of the cells was performed by flow cytometry with a FACSCalibur flow cytometer (BD Biosciences, NY, USA). FlowJo software was used for data analysis.
Cell cycle analysis
Cells were fixed in 70% ethanol at 4 °C overnight, and then the cells were washed twice with PBS and stained with propidium iodide at a final concentration of 50 ng/mL at room temperature for 30 min. Finally, the stained cells were analyzed by flowcytometry.
Enzyme-linked immunosorbent assay (ELISA)
Commercial ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to measure TNF-α and IL-10 concentrations. Culturing of the THP-1 cells was carried out using 6-well plates, pretreated with PMA for a period of 24 h, and then incubated with Ub (1–10 µg/mL) for 24, 48, and 72 h, respectively. Supernatants were collected and stored at −80 °C pending assay. ELISA assay was performed following the instructions of the manufacturer, and each of the samples was measured in triplicate. We calculated the TNF-α and IL-10 concentration via a standard curve.
Western blot
Ice-cold PBS was applied to wash the cells, and extracts were prepared with lysis buffer containing protease inhibitor. Lysates were subjected to SDS-PAGE and electrophoretically transferred to PVDF membranes. The membranes underwent blocking with 5% BSA at room temperature for 1 h and then underwent a period of incubation overnight with the primary antibody at 4 °C. The next day, the membranes were washed and incubated for 1 h with the second antibody conjugated to horseradish peroxidase at room temperature. The protein signal was detected by Fusion FX7 (VILBER, Paris, France).
Co-culturing of hepatoma cells with macrophages
1×106 THP-1 cells were seeded into polycarbonate membrane inserts (pore size, 0.4 µm; Corning, NY, USA) and pre-incubated with PMA to induce M0 macrophages. 24 h later, the M0 macrophages were treated with eUb (10 µg/mL) for 72 h. Meanwhile, 1×106 97H and HepG2.2.15 cells were seeded in a 6-well plate over a period of 24 h, and then the inserts were put into the 6-well plate to initiate co-culture. After 72 h of co-culturing, the 97H and HepG2.2.15 cells were collected for the following analysis.
Statistical methods
Statistical analysis was conducted out with GraphPad Prism. Data were expressed as mean ± SD. Data comparison was made with Student’s t-test as well as a one-way ANOVA test. P<0.05 was considered statistically significant.
Results
eUb had no significant effect on the proliferation and apoptosis of hepatoma cells
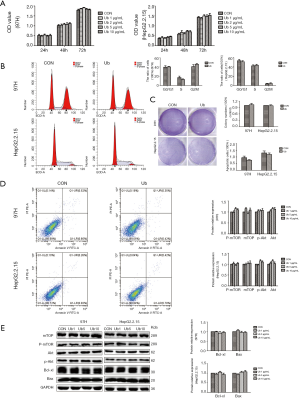
To understand the part of eUb released by the aged RBCs in the progression and recurrence of HCC, the influence of eUb in hepatoma cell (MHCC-97H and HepG2.2.15) proliferation and apoptosis was observed. 97H and HepG2.2.15 cells underwent treatment with different concentrations of eUb (1, 2, 5, 10 µg/mL) and were incubated over a period of 24, 48, and 72 h, respectively.
The CCK8 and cell cycle results showed that eUb did not affect the proliferation and cell cycle of 97H and HepG2.2.15 cells significantly (Figure 1A,B). The same result was obtained from the colony-formation assay (Figure 1C). In addition, the apoptosis of 97H and HepG2.2.15 cells after 72 h treatment with eUb (10 µg/mL) were detected by flow cytometry and the results showed that eUb did not alter the apoptosis of hepatoma cells (Figure 1D). Furthermore, we detected that key proteins were expressed in the Akt/mTOR signaling pathway which is influential in stimulating growth and proliferation in cells. Bcl-xl and Bax that were related to the cell apoptosis were also examined. The result showed that the total and phosphorylated protein levels of mTOR and Akt, Bcl-xl, and Bax were not changed simultaneously (Figure 1E). The above results demonstrated that eUb has no direct effect on hepatoma cell proliferation and apoptosis.
Hepatoma cell proliferation was not influenced after co-culturing with macrophages pretreated by eUb
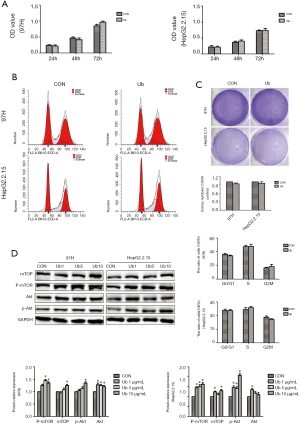
Since eUb exerts anti-inflammatory and immunoregulatory effects, and macrophage is reported to affect the growth and proliferation of cancer cells, we speculated that eUb might affect the proliferation of hepatoma cells via the involvement of macrophages. As the differentiation of human THP-1 monocytes has been commonly used as a model of human macrophages in vitro, we seeded THP-1 cells on inserts to differentiate into M0 macrophages through incubation along with phorbol 12-myristate 13-acetate (PMA). After 24 h, the THP-1 cells were adherent and then were treated with eUb (1, 5, 10 µg/mL) for 72 h. Afterward, the same amount of 97H and HepG2.2.15 cells were co-cultured with Ub-pretreated macrophages via indirect contact by Transwell inserts which allowed the exchange of soluble factors.
After 72 h of co-culturing, 97H and HepG2.2.15 cells were collected to measure their proliferation by CCK8 and colony-formation assays. The results showed that the involvement of the eUb-treated macrophages did not influence the proliferation and cell cycle of 97H and HepG2.2.15 (Figure 2A,B,C). However, it’s worth noting that mTOR and Akt abundance were both increased after co-culturing with macrophages pretreated by eUb (Figure 2D). These findings suggested that the co-culturing with macrophages pretreated by eUb activated the Akt/mTOR signaling pathway in hepatoma cells, which might be related to cell growth and proliferation.
Hepatoma cell apoptosis was inhibited after co-culturing with macrophages pretreated by eUb
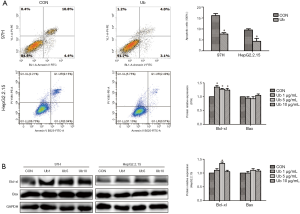
To further explore the pro-tumor effects of eUb on hepatoma cells via the involvement of macrophages, the apoptosis of 97H and HepG2.2.15 cells after co-culturing were detected. 97H and HepG2.2.15 cells were collected after being co-cultured with macrophages pretreated by eUb, and then flow cytometry and Western blotting were conducted to assess apoptosis in relation to hepatoma cells. The apoptosis of 97H and HepG2.2.15 cells were both decreased (Figure 3A), and Bcl-xl abundance was increased while no significant alteration of Bax abundance was observed in 97H and HepG2.2.15 cells after co-culturing with eUb-treated macrophages (Figure 3B). Collectively, we could draw a conclusion that eUb inhibited the apoptosis of hepatoma cells via the involvement of macrophages.
eUb modulated the phenotype and secretion function of macrophages
To explore the effect of eUb on macrophage specifically, we conducted a CCK8 assay to evaluate the influence of eUb on THP-1 monocyte proliferation, and we did not observe eUb to have a significant effect on monocyte growth (Figure 4A). However, we noticed the phenotype alteration of eUb-treated macrophages, which became elongated, flat and branching compared with the control group treated with PBS (Figure 4B).
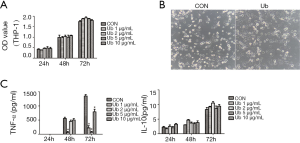
Tumor-associated macrophages (TAMs) are reported to modulate inflammatory and immune responses in the tumor microenvironment through cytokines secretion, such as pro-inflammatory cytokine TNF-α and anti-inflammatory cytokine IL-10. To examine the alteration of macrophage secretion upon eUb exposure, TNF-α and IL-10 in macrophage culture medium were measured by ELISA after eUb treatment. TNF-α was not detected after 24 h exposure while its concentration was up-regulated as the incubation time increased, and Ub inhibited TNF-α secretion compared with the control group. Meanwhile, IL-10 was increased with the incubation time, but Ub did not up-regulate IL-10 significantly (Figure 4C). Therefore, we speculated that eUb could modulate the phenotype and secretion function of macrophages.
Discussion
There is a few researches that has explored the biological effects of eUb in recent years, Singh et al. found eUb exerted cardioprotective effect by repressing cardiac myocyte apoptosis and myocardial fibrosis (17); eUb could mediate chemotaxis and modulate the phenotype and function of fibroblasts (18,19). eUb was also supposed to stimulate the proliferative ability of hepatectomized alcoholic liver cells in vivo (13). More importantly, Yan et al. verified that eUb might promote the acute lung infection-stimulated lung tumor metastasis (20); However, Job et al. confirmed that the eUb intravenous administration of up to 100 mg/kg did not display any toxic effects (21). These findings indicated the cell-type specific effects of eUb (22), and the effect of eUb would be influenced by the fundamental pathological state. The previous studies of our group have demonstrated that eUb could enhance the inhibiting effect of regulatory T cells on effector T cells (23) and stimulate Th2 cytokine IL-4 production while repressing Th1 cytokine IFN-γ (6). Meanwhile, we confirmed that eUb promoted lung tumor metastasis and reduced the long-term survival rate of melanoma mice (24). In this study however, we observed that eUb did not directly influence hepatoma cell proliferation and apoptosis. In view of the immunoregulatory effect of eUb, we speculated that eUb might modulate the function of immunocytes in the tumor microenvironment to influence the characteristics of hepatoma cells.
Recently, the transfusion of stored RBCs was demonstrated to exacerbate the sepsis-induced liver injury, which might be related to the activation of M1-polarized Kupffer cells (25). Therefore, the increased eUb in aged RBCs might modulate macrophage function to affect the progression and recurrence of HCC after stored RBC transfusion. From our results, the proliferation of hepatoma cells was not influenced while the Akt/mTOR signaling pathway in hepatoma cells was activated after co-culturing with the macrophages pretreated by eUb. In the meantime, the apoptosis of hepatoma cells was inhibited significantly after co-culturing. Since cancer development and progression rely on the tumor microenvironment, the cytokines released by macrophages after eUb treatment might provide a preferable microenvironment for the progression and recurrence of HCC.
Macrophages are soldiers of innate immunity and play an important role in tissue turnover, organ development, and regeneration (26,27). TAMs refer to the macrophages that infiltrate into tumor tissue and represent vital regulators of the complicated interaction between the immune system and cancer. Macrophages are plastic cells that can transform from one phenotype to another. Macrophages polarize toward “M1” (classically activated macrophages) or “M2” (alternatively activated macrophages) phenotype (28,29) in a manner dependent on their microenvironment. Induced by Th1 cytokines and microbial factors, M1 macrophages (M1s) secrete TNF-α, IL-12, and nitric oxide, promoting a pro-inflammatory response and mediating antimicrobial defense, and having an anti-tumor effect. While M2 macrophages (M2s) are induced by IL-13, IL-4 and glucocorticoids to secret arginase, TGF-β and IL-10, mediating inflammation resolution, angiogenesis, wound repair and tumor progression (28,30). In this study, we confirmed that eUb modulated the phenotype and secretion function of macrophages and eUb might enhance the carcinogenicity of hepatoma cells via the involvement of macrophages. Since eUb inhibited the concentration of TNF-α secreted by macrophages, we speculated that the extrinsic apoptosis signaling pathway of hepatoma cells might be affected after the co-culturing. Meanwhile, Bcl-xl abundance was increased which is a transmembrane molecule in the mitochondria and acts as an anti-apoptotic protein. Therefore, the intrinsic apoptosis pathway also could participate in the apoptosis initiated by the involvement of macrophages pretreated by eUb. However, ROS level, mitochondria membrane potential, the expression of cytochrome c and Apaf-1 should be detected to clarify the specific mechanism of Ub inhibiting cell apoptosis in the future study.
Conclusions
In this study, we demonstrated the pro-tumor potential of extracellular Ub in vitro. Act as a highly conserved protein in all eukaryotic cells, though eUb didn’t influence the proliferation and apoptosis of hepatoma cells directly, it still modulated the crucial biological properties of hepatoma cells by affecting the local microenvironment via the involvement of macrophages. However, our findings need to be demonstrated in vivo and further studies are needed to decipher the specific alterations of macrophages induced by eUb. In addition, we will use short- and long-time stored blood supernatant (with or without Ub depletion) to confirm the immunoregulation effect of eUb. The results of our study may provide some insights for removing the Ub released by aged RBCs to inhibit the progression and recurrence of HCC, thus improving the efficacy of aged RBCs transfusion for HCC patients.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This article does not contain any studies with human participants or animals performed by any of the authors.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shiba H, Ishida Y, Wakiyama S, et al. Negative impact of blood transfusion on recurrence and prognosis of hepatocellular carcinoma after hepatic resection. J Gastrointest Surg 2009;13:1636-42. [Crossref] [PubMed]
- Sugita S, Sasaki A, Iwaki K, et al. Prognosis and postoperative lymphocyte count in patients with hepatocellular carcinoma who received intraoperative allogenic blood transfusion: a retrospective study. Eur J Surg Oncol 2008;34:339-45. [Crossref] [PubMed]
- Shiba H, Ishida Y, Fujiwara Y, et al. Practice to minimize the use of blood products improve outcome after hepatic resection for hepatocellular carcinoma. Hepatogastroenterology 2013;60:1681-3. [PubMed]
- Wang D, Sun J, Solomon SB, et al. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion 2012;52:1184-95. [Crossref] [PubMed]
- Stapley R, Rodriguez C, Oh JY, et al. Red blood cell washing, nitrite therapy, and antiheme therapies prevent stored red blood cell toxicity after trauma-hemorrhage. Free Radic Biol Med 2015;85:207-18. [Crossref] [PubMed]
- Zhu X, Yu B, You P, et al. Ubiquitin released in the plasma of whole blood during storage promotes mRNA expression of Th2 cytokines and Th2-inducing transcription factors. Transfus Apher Sci 2012;47:305-11. [Crossref] [PubMed]
- Majetschak M, Zedler S, Hostmann A, et al. Systemic ubiquitin release after blunt trauma and burns: association with injury severity, posttraumatic complications, and survival. J Trauma 2008;64:586-96; discussion 96-8. [Crossref] [PubMed]
- Scofield SLC, Dalal S, Lim KA, et al. Exogenous ubiquitin reduces inflammatory response and preserves myocardial function 3 days post-ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2019;316:H617-28. [Crossref] [PubMed]
- Sixt SU, Dahlmann B. Extracellular, circulating proteasomes and ubiquitin - incidence and relevance. Biochim Biophys Acta 2008;1782:817-23. [Crossref] [PubMed]
- Buschow SI, Liefhebber JM, Wubbolts R, et al. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis 2005;35:398-403. [Crossref] [PubMed]
- Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res 2006;135:226-32. [Crossref] [PubMed]
- Hess JR, Sparrow RL, van der Meer PF, et al. Red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion 2009;49:2599-603. [Crossref] [PubMed]
- Sudzhashvili RS, Bakuradze ED, Modebadze IR, et al. Ubiquitin in combination with alcohol stimulates proliferative activity of hepatocytes. Georgian Med News 2013;86-90. [PubMed]
- Daino H, Matsumura I, Takada K, et al. Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood 2000;95:2577-85. [Crossref] [PubMed]
- Majetschak M, Krehmeier U, Bardenheuer M, et al. Extracellular ubiquitin inhibits the TNF-alpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood 2003;101:1882-90. [Crossref] [PubMed]
- Majetschak M, Cohn SM, Nelson JA, et al. Effects of exogenous ubiquitin in lethal endotoxemia. Surgery 2004;135:536-43. [Crossref] [PubMed]
- Singh M, Roginskaya M, Dalal S, et al. Extracellular ubiquitin inhibits beta-AR-stimulated apoptosis in cardiac myocytes: role of GSK-3beta and mitochondrial pathways. Cardiovasc Res 2010;86:20-8. [Crossref] [PubMed]
- Leiblein M, Ponelies N, Johnson T, et al. Increased extracellular ubiquitin in surgical wound fluid provides a chemotactic signal for myeloid dendritic cells. Eur J Trauma Emerg Surg 2020;46:153-63. [Crossref] [PubMed]
- Scofield SLC, Daniels CR, Dalal S, et al. Extracellular ubiquitin modulates cardiac fibroblast phenotype and function via its interaction with CXCR4. Life Sci 2018;211:8-16. [Crossref] [PubMed]
- Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res 2013;19:4706-16. [Crossref] [PubMed]
- Job F, Settele F, Lorey S, et al. Ubiquitin is a versatile scaffold protein for the generation of molecules with de novo binding and advantageous drug-like properties. FEBS Open Bio 2015;5:579-93. [Crossref] [PubMed]
- Steagall RJ, Daniels CR, Dalal S, et al. Extracellular ubiquitin increases expression of angiogenic molecules and stimulates angiogenesis in cardiac microvascular endothelial cells. Microcirculation 2014;21:324-32. [Crossref] [PubMed]
- Cao Y, Li C, Zhang Q, et al. Extracellular ubiquitin enhances the suppressive effects of regulatory T cells on effector T cell responses. Clin Lab 2014;60:1983-91. [Crossref] [PubMed]
- Zhang J, Chen S, Yan Y, et al. Extracellular Ubiquitin is the Causal Link between Stored Blood Transfusion Therapy and Tumor Progression in a Melanoma Mouse Model. J Cancer 2019;10:2822-35. [Crossref] [PubMed]
- Wu T, Wang L, An J, et al. Noninvasive Imaging of Stored Red Blood Cell-Transfusion Aggravating Sepsis-Induced Liver Injury Associated with Increased Activation of M1-Polarized Kupffer Cells. Shock 2017;48:459-66. [Crossref] [PubMed]
- Chang ZL. Recent development of the mononuclear phagocyte system: in memory of Metchnikoff and Ehrlich on the 100th Anniversary of the 1908 Nobel Prize in Physiology or Medicine. Biol Cell 2009;101:709-21. [Crossref] [PubMed]
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol 2009;9:259-70. [Crossref] [PubMed]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13. [Crossref] [PubMed]
- Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014;40:274-88. [Crossref] [PubMed]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787-95. [Crossref] [PubMed]


