
Effects of Tra2-beta1 on proliferation, apoptosis, and metastasis of hypoxic endometrial carcinoma cell and its correlation with clinicopathological features
Introduction
Endometrial carcinoma (EC) consists of a group of epithelial malignancies occurring in the endometrium, mainly in perimenopausal and postmenopausal women. The estimated incidence is at 15–20 per 100,000 women per year, making it the sixth most common cancer in women (1), and approximately 89% of cases occur between 65–69 years of age (2,3). Recently, the occurrence of EC has increased, and a trend towards younger patients has been observed (4). EC has a high incidence in developed countries, accounting for 6% of all cancers in females, with the incidence being highest in the United States and European countries (5). With the development of the economy and the change of diet structure, the incidence and mortality of EC in China are also gradually increasing, showing a rise in occurrence in younger patients and posing a serious threat to the health of women (6). It has been recognized that EC is related to many cancer-related genes. The occurrence and development of G protein-coupled estrogen receptor (GPER), estrogen receptor (ER), and human heterogeneous nuclear ribonucleoprotein G (hnRNP G) in EC are of great significance (7,8). These findings provide an important theoretical basis for the study of the diagnosis, prognosis, and targeted therapy of EC. It is thus of great significance to further deeply explore the role of cancer-related genes in EC for the diagnosis and treatment of this cancer.
Limited genes in vivo can generate protein diversely through alternative splicing of precursor mRNAs (9). The alternative splicing of mRNAs is determined by the interaction among the cis-acting elements, the trans-acting factors, and the family of serine/arginine (SR)-like proteins. transformer-2-beta1 (Tra2-beta1) is a member of the SR-like protein family and contains an RNA recognition motif (RRM) and two SR domains. When these acting elements are abnormal, they will lead to abnormal development of various diseases. Multiple experiments have demonstrated that Tra2-beta1 is highly expressed in tumor tissues and is associated with the prognosis, occurrence, development, and metastasis of tumors (10-12).
A large number of studies have shown that hypoxia exists during the formation and development of EC, and these hypoxic conditions will also seriously affect the development of EC (13). Firstly, hypoxia can promote the formation of tumor blood vessels in solid tumors and further promote the metastasis of solid tumors. Secondly, a hypoxic environment can enable tumor cells to eliminate the survival process and retain tumor cells with a strong ability to survive and divide, so as to improve the overall invasion and viability of tumor cells (14). In hypoxic tissues, the HIF-1 protein, which is closely related to the expression of vascular endothelial growth factor (VEGF), increases, thereby maintaining the function of cells under hypoxia (15,16). Tra2-beta1 has significant effects on the proliferation, apoptosis, metastasis, and cell cycle of tumor cells under a normal oxygen state. Whether Tra2-beta1 and Hif-1 are related to regulation, and the effects of Tra2-beta1 on hypoxic endometrial cells, remain to be studied.
We intended to elucidate the potential regulatory influence of alternative splicing of Tra2-beta1 in hypoxic EC cells and to determine its potential impact on the clinicopathological characteristics and clinical outcome.
Methods
Patients and tissue samples
A total of 128 consecutive patients with EC, who were treated at the Affiliated Hospital of Tongji University between January 2011 and December 2012, were included in this study. The study was approved by the ethics committee of the Affiliated Hospital of Tongji University, and the informed consent was obtained from all subjects. The age of patients at the time of diagnosis was 58.2±10.36 years (median ± interquartile range). All patients were diagnosed by pathological examination, who were not receiving hormone replacement therapy, surgery, chemotherapy, and radiotherapy. Those who lacked complete clinical data, or who had a history of secondary tumors, multiple organ failure, or multiple tumors, were excluded from this study. The general information and clinical pathological examination indicators were acquired through the hospital medical record inquiry system. Follow-up occurred every 4 weeks and was terminated on December 31, 2017.
Tissue samples were obtained along with surgery. Each patient specimen consisted of 1 pair of EC tissue and adjacent normal tissue (≥5 cm away from the tumor). The specimens of each patient were frozen by liquid nitrogen in order to detect Tra2-beta1 expression and were fixed by formalin for histopathological analysis.
RNA extraction from tissue and cDNA synthesis
Total tissue RNA was extracted by using the High Pure RNA kit (Tiangen Biotech, Beijing, China) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Tiangen Biotech, Beijing, China), according to the manufacturer’s instructions. An RNA quality check was performed by a photometer (accepted with A260/280 between 1.8 and 2.0).
Cellular RNA was extracted 48 h after transfection by applying the TRIzol reagent isolation protocol recommended by the manufacturer (Invitrogen, Carlsbad, CA, USA).
Real-time quantitative polymerase chain reaction (PCR)
cDNA was used as a template of qPCR at 95 °C for 10 min, for transgender degeneration at 95 °C for 15 s, and for annealing at 60 °C for 1 min (50 cycles). The dissolution curve analysis program was as follows: 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, and 60 °C for 15 s. For the primer sequence of Tra2-beta1, forward was ATGAGCGACAGCGGCGAGCA, and the reverse was TTAATAGCGACGAGGTGAGTA. For primer sequence of β-actin, forward was CCTGACTGACTACCTCATGAAG, and the reverse was GACGTAGCACAGCTTCTCCTTA. The qPCR reactions were performed in triplicate, and the comparative CT method (2−ΔΔCT method) was used to calculate the relative gene expression levels.
Western blot
Western blot analysis was performed to evaluate the Tra2-beta1 expression both in tissues and cells and before as well as after transfection. Proteins were extracted from cells, and the protein concentration was determined using a bicinchoninic acid (BCA) kit (Beyotime, China). After blocking, membranes (Millipore, USA) were incubated with milk containing the antibody (1:2,000 dilution; Abcam, USA), and then incubated overnight at 4 °C. Subsequently, horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:2,000 dilution; Abcam, USA) was added to the membranes and incubated for 1 h before detection.
Cell culture and transfection
Human EC cell line Ishikawa and HEC-1A were maintained in 89% Dulbecco’s Modified Eagle Medium (DMEM) medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 100 U/mL penicillin (Hyclone, USA) in a humidified atmosphere containing 5% CO2 at 37 °C. Cells (150×103/well) were placed in 6-well plates 24 h before transfection, leading to cell confluency of 70%. The Tra2-beta1siRNA sequence was provided by Genema Company (Shanghai, China) and is synthesized and purified by a Shanghai bioengineering cooperation. The sequence of siTra-beta1-1 (5'-3') was AGCTAAAGAACGTGCCAAT, siTra-beta1-2 (5'-3') was CCGATGTGTCTATTGTATA, siTra-beta1-3 (5'-3') was ACGCCAACCAGGAATTT, and scramble control siRNA (5'-3') was UGCAACUCACGGAAUCAUTT. The small interfering RNA (siRNA) with the highest knockdown rate was selected as the target siRNA in the combined intervention group and the knockdown group.
A hypoxia cell model built by cobalt chloride powder (17) was dissolved in 150 mL phosphate-buffered saline (PBS) solution, and the bacteria were removed by a 0.2-micron filter. At the logarithmic phase, 96 wells of cells were paved with plates, with 5,000 for each hole, and 160 µL of cobalt dichloride intervention solution was added to the culture for 24 h. In the combined interference group, Tra-beta1 was knocked down based on the hypoxia model.
Cell counting kit (CCK)-8 assays
A commercial CCK-8 (Sigma Chemical Co., St. Louis, MO, USA) assay was used to evaluate cell proliferation. Cells were seeded onto 96-well plates at a density of 5×103 cells per well and cultured at 37 °C in the air with 5% CO2. The absorbance was measured after an additional 3 h of incubation. A microplate reader (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to detect the absorbance at a wavelength of 450 nm.
Cell invasion assay
A cell invasion assay was performed using 6.5 mm transwells with 8.0 µm pore polycarbonate membrane inserts coated with a 0.5 mg/mL Matrigel matrix (BD, USA) placed in a 24-well plate (Corning, USA).
Statistical analyses
Statistical analyses were performed using SPSS (version 21; IBM Corp., Armonk, NY, USA). The student’s t-test was used for analyzing qPCR results, and western blot results with clinicopathological data. The correlation between Tra2-beta1 protein and EC clinicopathologic features in tissues was analyzed by one-way analysis of variance (ANOVA). Dunnett’s and least significant difference (LSD) tests were used for pairwise comparison. Patients’ survival curves were calculated using the Kaplan-Meier method. A log-rank test was performed to test the difference in survival curves in different groups. Multivariate prognostic analyses used multivariate Cox regression test in a forward stepwise manner; data are described as mean ± SD or mean, which were adjusted for Tra2-beta1 mRNA, and nuclear expression levels, age (<50 vs. ≥50), International Federation of Gynecology and Obstetrics (FIGO) Stage (I/II vs. III/IV), tumor differentiation grade (G1/G2 vs. G3), myometrial invasion, pathological types, lymph node metastasis, and estrogen receptor (ER) and progesterone receptor(PR). According to the median of 4.32 which was the relative expression level of Tra2-beta1 protein in EC, 128 EC patients were divided into two groups: the Tra2-beta1 high expression group (≥4.32) and the Tra2-beta1 low expression group (<4.32). When the data conformed to the normal distribution, it was described by the mean ± standard deviation; when it did not conform to the normal distribution, it was described by the median, interquartile range. A P value <0.05 was considered statistically significant.
Results
Tra2-beta1 protein expression in EC tissue and paracarcinoma tissue
The results of Western blot detecting the expression of Tra2-beta1 protein in EC and para carcinoma tissues are presented in Figure 1A. The relative expression level of Tra2-beta1 protein in EC tissues was significantly higher than that in para carcinoma tissues (P<0.001) (Figure 1B).
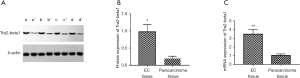
Tra2-beta1 RNA expression in EC tissue and para carcinoma tissue
qPCR detected the mRNA of Tra2-beta1 in EC tissue and para carcinoma tissue (Figure 1C). The expression of Tra2-beta1 mRNA in EC tissue and para carcinoma tissue (quartile) was 3.474 (0.931–9.872) and 1.031 (0.208–3.201), respectively. Mann-Whitney U test compared the differences between the two groups, and the results showed that the relative expression of Tra2-beta1 mRNA in EC tissues was significantly higher than that in adjacent tissues (P<0.01).
The correlation between Tra2-beta1 protein and EC patients’ clinicopathologic features
The results (Table 1) showed that Tra2-beta1 protein was highly expressed in patients with positive lymph node metastasis, high histologic grade, squamous cell carcinoma, high pathological stage, ER-positive, and PR positive (P<0.05). There was no significant correlation between Tra2-beta1 protein expression and patient's age, tumor size, and the depth of myometrial invasion.
Table 1
| Pathological features | Group | N (%) | Tra2-beta1 | Chi-square value | P | |
|---|---|---|---|---|---|---|
| High expression | Low expression | |||||
| Age | <50 | 38 (29.7) | 20 | 18 | 0.381 | 0.537 |
| ≥50 | 90 (70.3) | 42 | 48 | |||
| LN | P | 41 (32.0) | 28 | 13 | 19.144 | <0.001** |
| N | 87 (68.0) | 24 | 63 | |||
| Histological grade | G1 | 49 (38.3) | 12 | 37 | 8.268 | 0.016* |
| G2 | 69 (53.9) | 28 | 41 | |||
| G3 | 10 (7.8) | 7 | 3 | |||
| Myometrial invasion | Non | 22 (17.2) | 7 | 15 | 5.908 | 0.052 |
| Light | 77 (60.2) | 36 | 41 | |||
| Deep | 29 (22.7) | 19 | 10 | |||
| Pathological types | EAC | 98 (76.6) | 42 | 56 | 7.756 | 0.021* |
| ESAC | 21 (16.4) | 16 | 5 | |||
| Others | 9 (7.0) | 4 | 5 | |||
| FIGO | I stage | 87 (68.0) | 31 | 56 | 9.671 | 0.008* |
| II stage | 32 (25.0) | 19 | 13 | |||
| III stage | 9 (7.0) | 7 | 2 | |||
| Tumor size | <4 cm | 96 (75.0) | 45 | 51 | 1.500 | 0.221 |
| ≥4 cm | 32 (25.0) | 19 | 13 | |||
| ER | P | 105 (82.0) | 42 | 63 | 8.733 | 0.003* |
| N | 23 (18.0) | 17 | 6 | |||
| PR | P | 102 (79.7) | 44 | 58 | 11.739 | 0.001** |
| N | 26 (30.3) | 21 | 5 | |||
*, P<0.05; **, P<0.001. LN, lymph node metastasis; EAC, endometrioid adenocarcinoma; ESAC, endometrial adenoid squamous carcinoma; P, positive; N, negative.
The relationship between Tra2-beta1 protein and EC prognosis
For the 128 patients with EC, the median follow-up time was 32.5 (11.7–53.6) months. The median total survival time was 28.9 (10.3–49.2) months. The cumulative survival rates were 83.6%, 62.5%, and 52.3% for 1-, 3-, and 5-year survival, respectively. Kaplan-Meier analysis showed that the median survival time and the progression-free time were significantly longer in the Tra2-beta1 low expression group (survival time, 37 vs. 19 months; progression-free time, 31 vs. 13 months; low and high expression groups respectively; P<0.01).
The results of Cox regression showed that the independent risk factors included positive lymph node metastasis, histologic grade G3 and G2, pathological staging II, III period, ER, PR, and high Tra2-beta1 protein expression. Positive lymph node metastases, histologic grade G3, depth of muscular layer infiltration stage, pathological stage II and III, ER, PR, and high Tra2-beta1 protein expression were independent risk factors for the development of EC overall survival (Table 2).
Table 2
| Pathological features | Group | Progression-free survival | Total survival | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age | <50 | – | – | |||||
| ≥50 | 0.98 | 0.42–1.64 | 0.861 | 1.06 | 0.72–1.82 | 0.478 | ||
| LN | N | – | – | |||||
| P | 3.89 | 1.67–6.89 | 0.008* | 4.32 | 1.68–6.75 | 0.007* | ||
| Histological grade | G1 | – | – | |||||
| G2 | 2.12 | 1.09–3.98 | 0.047a | 1.08 | 0.64–2.85 | 0.361 | ||
| G3 | 2.56 | 1.23–4.23 | 0.023* | 1.98 | 1.09–3.68 | 0.042* | ||
| Myometrial invasion | Non | – | – | |||||
| Light | 1.35 | 0.78–2.78 | 0.671 | 1.45 | 0.73–2.92 | 0.285 | ||
| Deep | 2.09 | 0.87–4.28 | 0.187 | 1.89 | 1.08–2.89 | 0.048* | ||
| Pathological types | others | – | – | |||||
| ESAC | 1.46 | 0.87–3.76 | 0.372 | 1.28 | 0.98–3.98 | 0.062 | ||
| EAC | 0.98 | 0.46–2.98 | 0.792 | 0.83 | 0.47–1.89 | 0.398 | ||
| FIGO | Stage I | – | – | |||||
| Stage II | 2.78 | 0.93–2.78 | 0.058 | 3.09 | 1.32–3.95 | 0.011* | ||
| Stage III | 3.67 | 1.78–5.78 | 0.003* | 4.65 | 2.32–6.89 | <0.001** | ||
| Tumor size | <4 cm | – | – | |||||
| ≥4 cm | 1.65 | 0.75–3.29 | 0.321 | 1.87 | 0.89–4.09 | 0.078 | ||
| ER | N | – | – | |||||
| P | 3.64 | 2.65–7.83 | 0.000** | 2.98 | 2.13–6.43 | 0.001** | ||
| PR | N | – | – | |||||
| P | 2.31 | 1.56–8.34 | 0.002* | 1.78 | 1.21–4.68 | 0.031* | ||
| Tra2-beta1 | Low expression | – | – | |||||
| High expression | 2.89 | 1.15–6.43 | 0.043* | 3.21 | 1.98–8.56 | 0.003* | ||
*, P<0.05; **, P<0.001. HR, hazard ratio; CI, confidence interval; LN, lymph node metastasis; EAC, endometrioid adenocarcinoma; ESAC, endometrial adenoid squamous carcinoma; P, positive; N, negative.
Expression differences of Tra2-beta1 protein in 2 EC cell lines
Before transfection, we detected Tra2-beta1 protein expression in Ishikawa and HEC-1A by western blot. The results showed that the expression of the Tra2-beta1 protein in the Ishikawa was significantly higher than that of the HEC-1A cell line (Figure 2A).
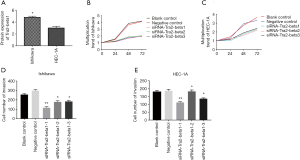
To verify our transfection effect, the expression level of Tra2-beta1 protein in the intervening cells was detected by western blot 48 h after transfection. The results showed that the expression of the Tra2-beta1 protein was significantly decreased in both Ishikawa and HEC-1A. In both types of cells, there was no significant difference in Tra2-beta1 protein expression between the blank control group and the negative transfection group.
The change of cell proliferation in each group (Figure 2)
After 72 h of Ishikawa cell culture, the number of cells (M ± SD) of the blank control group, the negative control group, siTra2-beta1-1, siTra2-beta1-2, and siTra2-beta1-3 were 8,345±67.3, 7,389±59.3, 4,568±56.8, 3,908±68.2, and 4,120±52.5, respectively. ANOVA and Dunnett-t-test showed significant differences in cell numbers between the five groups (P<0.001), with siRNA groups being lower than the negative group (P<0.001). There was no statistically significant difference in the number of cells between the negative control group and the blank control group (P>0.05). After 72 h of HEC-1A cell culture, the number of cells of the blank control group, the negative control group, siTra2-beta1-1, siTra2-beta1-2, and siTra2-beta1-3 was 6,321±18.3, 6,394±25.6, 3,362.3±25.9, 3,681±123.6, and 4,362±69.3, respectively. There were significant differences in the number of cells in the five groups (P<0.001), and the number of cells in the siRNA groups was significantly lower than that in the negative group (P<0.001). There was no statistically significant difference in the number of cells between the negative control group and the blank control group (P>0.05). The proliferation multiple at each time point was calculated based on the cell number at 0 h, and the proliferation trend was observed in all types of intervention cells. However, the proliferation trend was significantly decreased in cells transfected with siTra2-beta1. According to the number of cells at 0 h and the proliferation multiple at each time point, all types of intervention cells showed a proliferation trend, but the proliferation trend of cells transfected by siTra2-beta1 significantly decreased (Figure 2B,C).
Changes in cell invasiveness in each group (Figure 2)
The number of Ishikawa cells (M ± SD) in the blank control group, the negative control group, siTra2-beta1-1, siTra2-beta1-2, and siTra2-beta1-3 were 256±9.6, 298±10.9, 169±11.3, 178±12.4, and 183±9.7, respectively. In HEC-1A, the numbers were 179±9.4, 186±5.3, 112±4.5, 128±8.7, a 136±4.8. In Ishikawa and the HEC-1A cells, the results of variance analysis showed that the number of siRNA groups cells passing through the artificial basement membrane was lower than the negative control cells (P<0.05; Figure 2D,E).
The changes in cell cycle and apoptosis in each group
The fluorescence intensity of cells was measured by flow cytometry, and the DNA content and cell division stage were subsequently calculated (Table 3). ANOVA showed that the proportion of cells in the siRNA transfection group was increased in the G0/G1 stage, while it was decreased in the S stage for both Ishikawa and the HIC-1A cells. The apoptosis rate was significantly increased in both transfection groups.
Table 3
| Cell types | Groups | Cell population | Apoptosis rate (%) | ||
|---|---|---|---|---|---|
| G0/G1 stage | S stage | M/G2 stage | |||
| Ishikawa | Blank control | 52±4.3 | 32±5.4 | 16±2.6 | 0.73±0.12 |
| Negative control | 48±5.2 | 29±6.1 | 23±1.6 | 0.95±0.11 | |
| siTra2-beta1-1 | 62±6.1* | 28±4.3 | 10±3.5 | 1.69±0.13* | |
| siTra2-beta1-2 | 58±4.1* | 29±8.1 | 13±2.9 | 2.31±0.32* | |
| siTra2-beta1-3 | 64±9.2* | 25±2.4* | 11±3.7 | 2.61±0.26* | |
| HEC-1A | Blank control | 59±8.6 | 26±1.6 | 15±2.9 | 1.02±0.24 |
| Negative control | 62±7.3 | 28±3.7 | 10±1.6 | 1.24±0.31 | |
| siTra2-beta1-1 | 69±4.5* | 19±3.9* | 12±1.8 | 1.98±0.16* | |
| siTra2-beta1-2 | 74±6.1* | 21±2.8 | 5±1.7* | 2.36±0.28* | |
| siTra2-beta1-3 | 71±7.5* | 23±4.6 | 6±1.5* | 2.95±0.34* | |
*, P<0.05.
Proteins expression of Tra2-beta1, HIF-1a, and VEGF proteins in hypoxic cells
The expression levels of Tra2-beta1, HIF-1a, and VEGF proteins in the 4 groups of cells were detected by western blot after they were exposed to hypoxia or transfected with siTra2-beta1, or underwent both interventions (Table 4). HIF-1a and VEGF protein expression levels were increased in hypoxic cells. The expression levels of Hif-1a and VEGF protein were significantly decreased after transfection of the interfering siRNA with siTra2-beta1. In the combined intervention group, Tra2-beta1 protein was positively correlated with HIF-1α (correlation coefficient r=0.36, P<0.001) and VEGF protein (r=0.23, P<0.05). At the same time, there was a positive correlation between Hif-1a and VEGF protein expression level (r=0.59, P<0.001).
Table 4
| Groups | Tra2-beta1 (M ± SD) | HIF-1a (M ± SD) | VEGF (M ± SD) |
|---|---|---|---|
| Blank control | 1.08±0.13 | 1.12±0.19 | 1.13±0.14 |
| Hypoxia model | 1.89±0.09 | 5.46±1.45a | 3.87±0.32a |
| siTra2-beta1 | 0.22±0.04a,b | 0.97±0.09 | 1.06±0.13 |
| Combined intervention | 0.35±0.07b | 3.43±0.87b,c | 2.87±0.48b,c |
a, P<0.05 compared with the blank control group; b, P<0.05 compared with the hypoxia group; c, P<0.05 compared with the siTra2-beta1 group; combined intervention: built by cobalt dioxide and siRNA. M, mean; SD, standard deviation.
The proliferation capacity of intervention cells and hypoxia cells
After the transfection of siTra2-beta1, the cell optical density (OD) value was detected at 0, 24, 48 and 72 h, and the cell number was calculated according to the standard curve equation: y=0.00005x + 0.1602. The results of the variance analysis showed that the difference in cell number after 72 h in the 4 groups was statistically significant (P<0.001). Student-Newman-Keuls (SNK) comparison results showed that the proliferation of the hypoxia group built by cobalt dichloride was the fastest (P<0.001), followed by the proliferation of the siTra2-beta1 group (P<0.001). The joint intervention also exhibited the same phenomenon (P<0.001). According to the number of cells at time 0, the multiple proliferation at each time point was calculated. The results revealed that all types of intervention cells showed a proliferation trend, with faster proliferation in the hypoxia group compared to the siTra2-beta1 group and the combined intervention group (Figure 3A).

The capacity of invasiveness in hypoxia cells
The number of cells passing through the artificial basement membrane in the field under 200 times magnification was detected by Transwell assay to assess the invasiveness of cells in each group (Figure 3B). The number of cells in the blank control group, the hypoxia group, the siTra2-beta1 group, and the combined intervention group (mean ± SD) were 287±12.5, 421±16.8, 164±9.4, and 267±11.2, respectively.
The fluorescence intensity of cells was measured by flow cytometry, which speculated upon the DNA content and cell division stage (Table 5). The chi-square analysis showed that there were significant differences in the proportion of cells at all stages of the intervention in each group (P<0.001). The proportion of cells in the siTra2-beta1 group was higher in the G0/G1 stage and lower in the S stage and M/G2 stage compared to the control group. The hypoxia group was lower in the G0/G1 stage, and higher in the S stage and M/G2 stage compared to the control group. Similarly, compared with the hypoxia group, the combined intervention group had a higher proportion in the G0/G1 phase and a lower proportion in the S phase and M/G2 phase. At the same time, we compared the apoptosis rate of each group through variance analysis, and the results showed that the apoptosis rate of the 4 groups was statistically significant (P<0.001). The SNK test comparison results show that Tra2-beta1 had the highest apoptosis rate of 2.56±0.17. In contrast, hypoxia cells had the lowest apoptosis rate of 0.45±0.03.
Table 5
| Groups | Stage (%) | Apoptosis rate (%) | ||
|---|---|---|---|---|
| G0/G1 stage | S stage | M/G2 stage | ||
| Blank control | 52 | 32 | 6 | 0.87±0.14 |
| Hypoxia model | 34 | 46 | 20 | 0.45±0.03a |
| siTra2-beta1 | 69 | 24 | 7 | 2.56±0.17a |
| Combined intervention | 47 | 36 | 13 | 1.28±0.16b,c |
a, P<0.05 compared with the blank control group; b, P<0.05 compared with the hypoxia group; c, P<0.05 compared with siTra2-beta1 group.
Discussion
This study explored the relationship between the occurrence and development of EC by examining the expression characteristics of Tra2-beta1 in EC tissues and performed functional in vitro experiments on TRA-Beta1-knocked-down cells and hypoxic model cells. The results showed that the high expression of Tra2-beta1 in EC was significantly correlated with the clinical characteristics of patients and closely related to the occurrence and severity of EC. Furthermore, we constructed a hypoxic cell model that promoted proliferation, apoptosis, and metastasis. Knockout of Tra2-beta1 in hypoxic EC cells inhibits the metastasis of EC cells, promotes apoptosis, and keeps cells in the G0/G1 phase, slowing their division and growth. The findings of this study suggest that the development of EC is positively correlated with Tra2-beta1, and Tra2-beta1 may reverse the effects of hypoxia in EC.
In the process of formation and development of most EC, hypoxic conditions arise and affect the development of EC. Therefore, in order to further analyze the correlation between Tra2-beta1 and HIF-1a/VEGF signals, we analyzed the correlation between Tra2-beta1, HIF-1a, and VEGF expression levels in hypoxic EC cells. Our study showed that knockdown of Tra2-beta1 in hypoxic EC cells could inhibit the metastasis of EC cells, promote apoptosis, and keep cells in the G0/G1 phase, slowing their division and growth, enhancing the proliferation and metastasis capacity of hypoxic cancer cells, and reducing the apoptosis capacity.
HIF-1a is an oxygen stress factor that is highly expressed in hypoxia cells and has been increasingly recognized as playing a broad and critical role in normal development, postnatal physiology, cancer, and many other diseases (18-20). HIF-1a may be associated with EC based on the C1772T polymorphism (21). Horrée’s results showed that HIF-1a was not a risking factor for EC, although this study still maintains that HIF-1a promotes EC growth (22).
VEGF is an important angiogenic factor, which can promote the formation of new blood vessels and lymphatic vessels, and thus plays an important role in the progress of EC. It is a vital element in tumor proliferation and several pathological processes (23). It has been reported that VEGF takes part in the tumorigenesis and metastasis of EC (24). Moreover, VEGFRs were shown to be highly expressed in EC, with VEGFR-3 being remarkably correlated with tumor stage and survival (25).
Our previous study hinted that HIF-1a and VEGF had a higher positive rate in patients with positive lymph node metastasis, high histological grade, tumor size greater than 4 cm, and positive PR. Taking all evidence into account, we believe that Tra2-beta1 is involved in some way with the regulation of HIF-1a expression; the best proof for this theory is that the expression of Tra-beta1 is positively correlated with the expression of Hif-1a and VEGF, which have an important role in EC development.
This study did not examine the VEGF, and HIF-1a proteins would change after the knockdown of Tra-beta1, and what specific pathways might be used. Furthermore, we did not analyze the effect of VEGF and HIF-1a on the prognosis and development of patients. These are all areas that we should investigate in the future.
Conclusions
The present study strongly supports the potential clinical significance of Tra2-beta1 in EC prognosis. The activity of Tra2-beta1 might critically influence the prognosis process of EC. The present findings provide insight into the pharmaceutical targeting therapy and prognosis of EC, and our study offers new ideas and approaches to the occurrence and development of EC.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.66). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics committee of the Affiliated Hospital of Tongji University, and the informed consent was obtained from all subjects. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Stanojevic Z, Djordjevic B, Todorovska I, et al. Risk factors and adjuvant chemotherapy in the treatment of endometrial cancer. J BUON 2008;13:23-30. [PubMed]
- Ryan AJ, Susil B, Jobling TW, et al. Endometrial cancer. Cell Tissue Res 2005;322:53-61. [Crossref] [PubMed]
- Rodríguez-Mias NL, Cortes J, Xercavins J, et al. Current situation: lower genital tract pathology and colposcopy training in spanish gynecology and obstetrics residents. J Low Genit Tract Dis 2013;17:12-6. [Crossref] [PubMed]
- Huang C, Xie MH, Liu W, et al. A structured RNA in hepatitis B virus post-transcriptional regulatory element represses alternative splicing in a sequence-independent and position-dependent manner. FEBS J 2011;278:1533-46. [Crossref] [PubMed]
- Song Y, Liao YM. Expressions of ER, PR and p53 in young patients with endometrial carcinoma and clinical significance. Chin J Modern Med 2017;27:47-52.
- Hirschfeld M, Ouyang YQ, Jaeger M, et al. HNRNP G and HTra2-beta1 regulate estrogen receptor alpha expression with potential impact on endometrial cancer. BMC Cancer 2015;15:86. [Crossref] [PubMed]
- Yang M, Li L, Wang J, et al. Heterogeneous nuclear ribonucleoproteins (hnRNPs) and human transformer-2-beta1 (hTra2-beta1)-regulated estrogen receptor-alpha improves prognosis of endometrial cancer. Eur J Gynaecol Oncol 2014;35:701-7. [PubMed]
- Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010;463:457-63. [Crossref] [PubMed]
- Kuwano Y, Nishida K, Kajita K, et al. Transformer 2β and miR-204 regulate apoptosis through competitive binding to 3' UTR of BCL2 mRNA. Cell Death Differ 2015;22:815-25. [Crossref] [PubMed]
- Ji L, Ni T, Shen Y, et al. Transformer 2β (Tra2β/SFRS10) positively regulates the progression of NSCLC via promoting cell proliferation. J Mol Histol 2014;45:573-82. [Crossref] [PubMed]
- Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol 2008;198:218.e1-6. [Crossref] [PubMed]
- Diakos CI, Charles KA, Mcmillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Kim KW, Myers CJ, Jung DK, et al. NVP-BEZ-235 enhances radiosensitization via blockade of the PI3K/mTOR pathway in cisplatin-resistant non-small cell lung carcinoma. Genes Cancer 2014;5:293-302. [PubMed]
- Miyasaka A, Oda K, Ikeda Y, et al. PI3K/mTOR pathway inhibition overcomes radioresistance via suppression of the Hif-1α/VEGF pathway in endometrial cancer. Gynecol Oncol 2015;138:174-80. [Crossref] [PubMed]
- Su XJ, Hu JT, Geng J, et al. Expression of Hif-1α and VEGF in endometrial carcinoma and its correlation with tumor angiogenesis. Hainan Med J 2016;27:3828-31.
- Wu YY, Liu HJ, Qiao FY. Effects of cobalt dichloride hypoxia on apoptosis and invasion of trophoblastic cells . Reprod Contraception 2009;29:354-9.
- Kazi AA, Koos RD. Estrogen-induced activation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 2007;148:2363-74. [Crossref] [PubMed]
- Quintero M, Mackenzie N, Brennan PA. Hypoxia-inducible factor 1 (HIF-1) in cancer. Eur J Surg Oncol 2004;30:465-8. [Crossref] [PubMed]
- Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer 2006;13:739-49. [Crossref] [PubMed]
- Kafshdooz L, Tabrizi AD, Mohaddes SM, et al. The polymorphism of hypoxia-inducible factor-1a gene in endometrial cancer. Asian Pac J Cancer Prev 2014;15:10393-6. [Crossref] [PubMed]
- Horrée N, Groot AJ, van Hattem WA, et al. HIF-1A gene mutations associated with higher microvessel density in endometrial carcinomas. Histopathology 2008;52:637-9. [PubMed]
- Lohela M, Bry M, Tammela T, et al. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 2009;21:154-65. [Crossref] [PubMed]
- Mahecha AM, Wang H. The influence of vascular endothelial growth factor-A and matrix metalloproteinase-2 and -9 in angiogenesis, metastasis, and prognosis of endometrial cancer. Onco Targets Ther 2017;10:4617-24. [Crossref] [PubMed]
- Wang J, Taylor A, Showeil R, et al. Expression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and related proteins in endometrial carcinoma. Cytokine 2014;68:94-100. [Crossref] [PubMed]


