
Pancreatic cancer patients with germline BRCA mutations can benefit from olaparib treatment
Pancreatic cancer, which is one of the leading causes of cancer death in the United States, can be initiated by many factors, including mutations in BRCA1 and BRCA2. The majority of familial pancreatic cancers are caused by these mutations. For individuals with a BRCA2 mutation, the lifetime risk of developing pancreatic cancer is 5–10% (1-3).
It has long been known that, because of deficiencies in homologous recombination repair, patients with BRCA mutations respond well to chemotherapeutic agents that bind to DNA directly and induce double-strand breaks to cause their cytotoxic effect (4). The BRCA mutation prevents tumor cells from repairing the double-strand damage caused by these chemotherapeutic agents. Accumulated DNA damage in the tumor cells ultimately eliminates the tumor. PARP inhibitors are a group of drugs that inhibit cells from repairing double strand breaks (5), and they are used in platinum therapy for cancer. Olaparib (AZD2281) is a powerful oral PARP inhibitor that induces toxicity for BRCA1/2-deficient tumor cells (6).
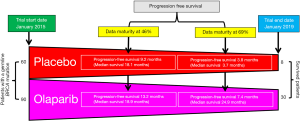
In a recent paper, “Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer,” Golan et al. (2019) screened 3,315 patients with pancreatic cancer and found that 247 of them had a germline BRCA mutation. All 3,315 patients received platinum-based chemotherapy before this study was conducted. Out of the 247 patients with a germline BRCA mutation, 154 were selected for randomized study. Ninety patients were given 300 mg olaparib orally twice a day, and 61 patients were given placebo (7). The authors found no difference in overall median survival between the olaparib (18.9 months) and placebo (18.1 months) groups at a data maturity of 46% (Figure 1). However, at this interim data analysis stage, the median disease-free progression time was longer in the olaparib group (13.2 months) than in the placebo group (9.2 months). Importantly, the median progression-free survival was significantly longer (P=0.004) in the olaparib group (7.4 months) than in the placebo group (3.8 months) at a data maturity of 69%. At the closing of this study (January 15, 2019), 30 patients in the olaparib group and 8 from the placebo group were still on the treatment regime. Moreover, two patients in the olaparib group had a complete response. The authors reported that the duration of median response for the olaparib group was 24.5 months. By contrast, Kaufman et al. (2015) found that, when patients with pancreatic cancer were given 400 mg olaparib orally twice a day, the duration of median response was 134 days (8). Significantly, olaparib was most effective in increasing the progression-free survival of metastatic pancreatic cancer patients with a germline BRCA mutation.
Conclusions
Unhindered, pancreatic cancer will soon be the second leading cause of cancer-related death. Despite the recent advances in surgery, chemotherapy, radiotherapy, and targeted therapies, pancreatic cancer continues to have a 5-year survival rate of less than 10%. Immunotherapy has demonstrated efficacy in treating several types of solid tumors; accordingly, there has been great interest in the role of immune cells in pancreatic cancer and applying various immunotherapeutic approaches, but most trials currently remain negative. Although pancreatic cancer is distinguished by prominent desmoplasia (fibrosis), its microenvironment is enriched with immune cells. Despite the presence of many immune cells in pancreatic cancer, immune dysfunction is observed in patients with pancreatic cancer where the tumor microenvironment is immunosuppressive, which thus inhibits the activation or function of immune effectors. Therefore, identifying novel combinational approaches that can overcome immune signaling suppression in response to genotoxic cancer therapeutic agents is required. In order to develop effective combination therapies, it is imperative to understand multi-modal interactions between therapeutic agents. Golan et al. (2019) show that a population of pancreatic cancer patients with germline BRCA mutations can achieve longer progression-free survival with olaparib treatment. It would be important in the future to understand the roles of olaparib in overcoming the immune signaling suppression microenvironment of germline BRCA-mutant pancreatic cancer.
Acknowledgments
We thank Dr. Jonathan Feinberg for editing this manuscript.
Funding: The research in the authors’ laboratory is supported by
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferrone CR, Levine DA, Tang LH, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol 2009;27:433-8. [Crossref] [PubMed]
- van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, et al. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet 2005;42:711-9. [Crossref] [PubMed]
- Pilarski R. The Role of BRCA Testing in Hereditary Pancreatic and Prostate Cancer Families. Am Soc Clin Oncol Educ Book 2019;39:79-86. [Crossref] [PubMed]
- Kim R, Byer J, Saif MW. BRCA and pancreatic cancer: selection of chemotherapy. JOP 2012;13:180-1. [PubMed]
- Malyuchenko NV, Kotova EY, Kulaeva OI, et al. PARP1 Inhibitors: antitumor drug design. Acta Naturae 2015;7:27-37. [Crossref] [PubMed]
- Evers B, Drost R, Schut E, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res 2008;14:3916-25. [Crossref] [PubMed]
- Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019;381:317-27. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]


