
Combination therapy of first and third generation EGFR-TKIs for an advanced lung adenocarcinoma patient harboring EGFR mutations and amplification: a case report
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have opened up a new era that improving the prognosis in patients with non-small cell lung cancer (NSCLC). And it’s generally recommended in first-line treatment by NCCN guidelines for those advanced NSCLC who diagnosed with EGFR driver mutation. Despite the remarkable responses of first-generation EGFR-TKI treatment, disease progression inevitably occurs after approximately 9–13 months of these targeted therapies (1-3). Among the mechanisms acquired during EGFR-TKI resistance, 60% of the cases may develop secondary EGFR alterations such as T790M point mutation alone, T790M with EGFR amplification and other EGFR point mutations. The occurrence of developing simultaneous T790M mutation and EGFR amplification accounts for 10% (4). However, in the situation of complex molecular alterations, switching of EGFR-TKI alone may be insufficient to provide desired efficacy. Therefore, next-generation inhibitors or combination therapy options need to be explored. Here, we presented a successful case of stage IV EGFR-mutated NSCLC patient with acquired T790M mutation and EGFR amplification after first-line treatment who received combination therapy of osimertinb and icotinib to achieve progressive-free survival (PFS) of more than 2 years. We present the following case in accordance with the CARE-Guideline.
Case presentation
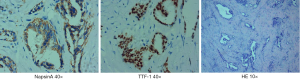
A 74-year-old male presented to our clinic with cough, thinness and dyspnea during or after exercise on September 3rd 2015. He had no past personal medical history and denied family history of lung cancer. Chest enhanced computed tomography (CT) showed an irregular mass in the left upper lobe with the maximum size of 9.2×6.6 cm, enlargement of hilar and mediastinal lymphadenopathy with a large amount of pleural effusion on the left side. Medical thoracoscopy showed small nodules of varying sizes in the left pleura. The left pleural pathology was adenocarcinoma which indicates metastasis. Taken together, the patient was diagnosed with left lung adenocarcinoma stage IV (cT4N3M1). Meanwhile, the patient underwent mutation analysis by amplification refractory mutation system (ARMS) method. The result revealed exon 19 deletion in EGFR.
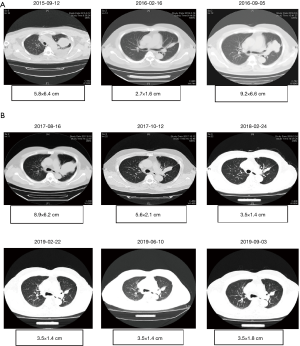
The patient was treated with first-generation EGFR-TKI icotinib at 125 mg once daily orally from Sep 22nd 2015. This resulted in the relief of cough and dyspnea. In Feb 2016, the reexamination of chest CT revealed a significant reduction in lesions [very good partial response (VGPR)] base on the criteria in Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. But stated from Sep 2016, the reexamination of chest CT showed lesion enlargement with PFS of 12 months (Figure 1). Then the patient was given three cycles of chemotherapy with pemetrexed and nedaplatin in addition to icotinib. Reexamination of chest CT showed stable lesion. But the chemotherapy was discontinued due to the intolerance of nausea and vomiting associated with the therapy.
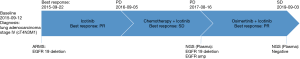
On Aug 16th 2017, the patient experienced progressive disease (PD) upon observation of enlargement of pulmonary lesions from CT scans. In order to specify the acquired resistance of target therapy, liquid biopsy with next-generation sequencing (NGS) was performed. NGS sequencing of plasma DNA at PD was performed using a panel consisting of 12 genes (Shanghai Tongshu Biotechnology Co., Ltd., Shanghai, China). The results revealed EGFR exon 19 deletion (64.61%), T790M point mutation (29.74%) and EGFR amplification (CN =5.47). Based on the NGS results, the patient was treated with the combination of icotinib (125 mg once daily) and osimertinib (80 mg once daily) from Sep 2017. Within 1-month, significant lesion reduction was achieved base on chest CT data (5.6×2.1 cm). Continually reexamination of chest CT from Feb 2018 to the last follow-up on Sep 2019 indicated that the patient continued to benefit from the combination therapy and his lung lesions remained stable (Figure 2). The patient had been in a good mental state with physical mobility evaluated as ECOG PS score 0 without adverse effects. Also, a liquid rebiopsy in Mar 2019 was performed. Targeted NGS of 168 cancer-related genes (Burning Rock Biotech, Guangzhou, China) was performed on DNA derived from plasma, revealing that the ctDNA was negative. In total, Figure 3 summarizes the clinical history of the patient including treatment received, objective responses (OR) as well as mutation profile detected from the patient samples during the course of the treatment.
Discussion
EGFR-targeted therapy has become standard of care in advanced stage EGFR-mutant NSCLC with three generations of EGFR-TKI now available on the market. In this case, we report the simultaneous presence of two resistance mechanisms (EGFR T790M point mutation and EGFR amplification) with the original mutation of EGFR exon 19 deletion after first-line EGFR-TKI treatment. Although multiple resistance mechanisms have already been identified and different combination therapy schemes have been suggested and tested, there is rare clinical evidence of first-generation in combination with third-generation EGFR-TKI in NSCLC.
The patient with NSCLC in this case responded to first-line EGFR-TKI icotinib with PFS of 12 months. Genetic testing after progression suggests that there are still EGFR exon 19 deletion along with EGFR amplification and T790M point mutation. With the breakthrough designation and approval of third-generation EGFR-TKI osimertinib in Dec 2016, the survival of patients who acquired T790M mutation after initial TKI treatment has been extended by osimertinib with mPFS of 9.6 months (5). However, multiple studies have shown that EGFR amplification is one of the resistance mechanisms of the third-generation EGFR-TKIs (6,7), which suggests that osimertinib alone may not be fully effective if EGFR amplification occurs simultaneously with EGFR exon 19 deletion and T790M point mutation. On the other hand, icotinib showed significant efficacy for patients harboring EGFR overexpression or amplification although the study was done in advanced esophageal squamous cell carcinoma, suggesting the ability of icotinib to control EGFR overexpression or amplification (8). Therefore, combination therapy of icotinib and osimertinib which target EGFR amplification and EGFR T790M, respectively, was administered. The follow-up NGS detection of blood after combination therapy was negative, indicating that the disease was well controlled under the two drugs. The overall survival time of the patient was significantly prolonged for more than 2 years, and the quality of life is high without obvious discomfort for this patient.
There is a certain limitation to this case study. It is debatable whether monotherapy of osimertinib could also bring favorable response to this patient. However, compared with the mPFS (9.6 months) in patients progressed after first- or second-generation EGFR-TKI who were treated with osimertinib alone, this patient achieved PFS over 2 years after progression. It is likely that combination therapy of first- and third-generation EGFR-TKIs might underlie the markedly prolonged and high-quality survival.
With new treatment regimens and the help from continuous disease monitoring by genetic testing, the treatment of advanced NSCLC has become more precise and personalized. We believe that the combination strategy proposed in this case is worth consideration and warrants further studies to support it.
Conclusions
This case report provides clinical evidence of the safety and efficacy of combined inhibition of EGFR T790M mutations and EGFR amplification in advance adenocarcinoma lung cancer patient using a combination therapy of osimertinib and icotinib. If other patients come across with a similar situation, this treatment strategy could be explored as an option. Moreover, we propose that monitoring of molecular mutation profiling using liquid biopsy or re-biopsy samples is of clinical importance, which could guide treatment options in EGFR-TKI-treated NSCLC patients.
Acknowledgments
The authors thank both the patients and his family. They also thank Burning Rock Biotech for performing NGS sequencing, as well as the investigators, study coordinators, operation staff and the whole project team who worked on these cases.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 2017;28:2443-50. [Crossref] [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Knebel FH, Bettoni F, Shimada AK, et al. Sequential liquid biopsies reveal dynamic alterations of EGFR driver mutations and indicate EGFR amplification as a new mechanism of resistance to osimertinib in NSCLC. Lung Cancer 2017;108:238-41. [Crossref] [PubMed]
- Kim TM, Song A, Kim DW, et al. Mechanisms of acquired resistance to AZD9291: a mutation-selective, irreversible EGFR inhibitor. J Thorac Oncol 2015;10:1736-44. [Crossref] [PubMed]
- Huang J, Fan Q, Lu P, et al. Icotinib in patients with pretreated advanced esophageal squamous cell carcinoma with EGFR overexpression or EGFR gene amplification: a single-arm, multicenter phase 2 study. J Thorac Oncol 2016;11:910-7. [Crossref] [PubMed]


