miR-28-5p inhibits carcinogenesis in colon cancer cells and is necessary for erastin-induced ferroptosis
Introduction
Colorectal cancer (CRC) is an extremely common form of gastrointestinal disease. In developed countries, CRC claims the second highest number of adult lives of any cancer (1). For CRC patients, especially those diagnosed at later stages, chemotherapy is the most common treatment However, in the clinical setting, many chemotherapeutic drugs, including cisplatin and oxaliplatin, are associated with acquired resistance and toxicity, which lead to failure in cancer patient management (2,3). Therefore, a new approach which can overcome the issues posed by chemotherapeutic drug resistance and toxicity is desperately needed to improve treatment for patients with CRC.
Ferroptosis, a recently identified form of regulated cell death, occurs when iron-dependent lipid peroxide accumulates, thus employing a different method to those of apoptosis and necrosis (4,5). Ferroptosis plays a crucial role in the pathogenesis of a number of diseases, including cancer (6,7). Therefore, triggering ferroptosis might offer a novel therapeutic strategy for treating such diseases. A previous study showed that ferroptosis helped to facilitate RSL3-induced cell death in CRC cells, and is perhaps a pervasive and dynamic form of cell death in cancer therapy (8). However, the underlying mechanisms of ferroptosis are largely unknown and need to be further illuminated.
miRNAs are single-stranded, small non-coding RNA molecules that can be oncogenic or can take up a tumor-suppressive role and are known to participate in the initiation, progression, and metastasis of various types of cancer (9). miR-28-5p has been shown to target a number of cancer-associated genes and participates in cancer cell proliferation, migration, invasion, and epithelial-mesenchymal transition (10). A previous investigation demonstrated that in comparison with normal colon samples, CRC samples exhibited downregulation of miR-28-5p, and that the expression levels of cyclin D1 (CCND1) and homeobox B3 (HOXB3) are inhibited when miR-28-5p is overexpressed (11). However, the role of miR-28-5p in ferroptosis in CRC cells is not yet understood. In the current study, we explored the effect of erastin, an inducer of ferroptosis, in colon cancer cells and examined the role of miR-28-5p in colon cancer cells undergoing ferroptosis, with the aim of revealing the regulatory mechanism of miRNAs in ferroptosis in CRC cells.
Methods
Cell culture
The human colon cancer lines HCT116 and SW480 and the human colon line NCM460 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Roswell Park Memorial Institute (RPMI)-1640 (Gibco, Grand Island, NY, USA) medium containing 10% fetal bovine serum (FBS; Gibco) and 100 U/mL penicillin-100 µg/mL streptomycin (Solarbio, Beijing, China) were used for cell culture. All cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Iron, malondialdehyde (MDA), and glutathione (GSH) concentration and glutathione peroxidase (GPX) vitality assay
An Iron Assay Kit (Baiaolaibo, Beijing, China) was used to measure relative iron concentration in cell lysates. The MDA concentration was detected with a Lipid Peroxidation Assay Kit (Sigma, St Louis, Mo, USA). A Glutathione Assay Kit (Solarbio, Beijing, China) was employed to assess the GSH concentration. GPX vitality was assessed by GPX vitality assay (Leagene, Beijing, China). All assays were performed according to the manufacturer’s instructions.
Transfection of miR-28-5p mimics and inhibitors
The effect of miR-28-5p on HCT116 cells was evaluated by transfecting miR-28-5p mimics or inhibitors purchased from Bioneer (Shanghai, China). Cells were plated with RPMI-1640 with 10% FBS without antibiotics in 6-well plates at a density of 2×105 cells/well for 24 h. Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) was then used to transfect the cells in accordance with the manufacturer’s protocol. miR-28-5p mimics and miR-28-5p inhibitors were used at final concentrations of 80 and 100 pM, respectively, in antibiotic-free Opti-minimum essential medium (Opti-MEM, Gibco, Grand Island, NY, USA). The medium was discarded and replaced with RPMI-1640 (10% FBS, 20 µmol/L erastin) 6 h later. The extraction of total RNA and protein was performed 48 h after transfection.
Cell viability assays
CCK8 assays were conducted to evaluate cell viability. Briefly, HCT116 cells were seeded at 104 cells/well in 96-well plates in 0.1 mL RPMI-1640 for 12, 24, and 48 h. At each time point, 10 µL of CCK8 solution (5 mg/mL) was added, before the cells were incubated for 2 h at 37 °C. A microplate reader (Eppendorf, Hamburg, Germany) was used to read the absorbance was read at 490 nm.
Cell apoptosis assays
Flow cytometry was conducted to assess apoptosis. The Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (BD, San Jose, CA, USA) was used to measure the rate of apoptosis. Trypsin [without ethylene diamine tetraacetic acid (EDTA)] was used to collect cells, which were centrifuged at 1,000 ×g for 5 min at room temperature. Next, the cells were washed twice with phosphate buffer saline (PBS), resuspended in 100 µL Annexin V-FITC Binding Buffer, and mixed with 5 µL Annexin V-FITC and 5 µL propidium iodide. The cells were subsequently stained for 15 min in darkness. Then, 400 µL of Annexin V-FITC Binding Buffer was added and the cells were kept in the dark and examined by flow cytometry. Cell Quest™ (BD) was used to analyze the results.
Migration and invasion assays
Transwell™ assays (Corning, NY, USA) were used to measure the migratory and invasive abilities of HCT116 cells. To coat the surface of the polycarbonate membrane, serum-free medium, with or without the addition of 50 µL Matrigel™ (Corning) was used to wash the upper chamber of the Transwell, into which cells (105) in 0.2 mL of serum-free RPMI-1640 were seeded. Meanwhile, 0.5 mL of RPMI-1640 supplemented with 10% fetal bovine serum was put into the lower chamber. The membrane was incubated at 37 °C for 48 h, before the removal of cells from the upper chambers. Finally, crystal violet was used to stain the invaded cells in the lower chambers, and they were observed and counted using a high-power microscope.
Western blotting assays
For Western blotting analysis, radio immunoprecipitation assay (RIPA) lysis buffer (100 µL) was used to extract the lysates of total cellular proteins. Next, 50 µg of lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electroblotting was performed to transfer them to nitrocellulose membranes. The membranes were blocked for 1 h with 5% blocking solution before being incubated overnight at 4 °C with primary antibodies purchased from Abcam Biotechnology [N4BP1: 1:2,000 dilution; glyceraldehyde 3-phosphate dehydrogenase (GAPDH): 1:5,000 dilution]. The membranes were then washed with tris buffered saline tween (TBST) three times and incubated with goat anti-rabbit IgG secondary antibodies (1:3,000; Abcam, Cambridge, UK) for 1 h. Immunoreactivity was measured using a two-color IR imaging system (Odyssey, Lincoln, USA).
Quantitative real-time polymerase chain reaction (RT-PCR)
The extraction of total cellular RNA was performed using 1 mL Nuclezol™ Reagent (Macherey-Nagel, Wiesbaden, Germany) in line with the instructions of the manufacturer. Then, 3.75 µL of RNA was reverse-transcribed to cDNA in a 10-µL reaction using a micRNA Reaction kit (Takara, Tokyo, Japan). A 7500 Real-Time PCR system (Applied Biosystems, Fostercity, CA, USA) was employed to carry out RT-PCR. The PCR program was as follows: 40 cycles of 95 °C for 30 s, 95 °C for 5 s, and 60 °C for 34 s. Each experiment was performed in triplicate. Gene expression was normalized to that of U6.
Primer sequences
The primer sequences of miR-28-5p were: forward 5'-CGC AAG GAG CTC ACA GTC TAT TGA G-3'; reverse: mRQ3'primer (Takara, Tokyo, Japan). The primer sequences of N4BP1were: forward 5'-CAC CTT CTG TTG CCT CTC CAA GTC-3'; reverse 5'-GGG TTC TGG CTG GTG TAA AC-3'.
Statistical analyses
All statistical analyses were performed using SPSS 17.0 (IBM Corp, Armonk, NY, USA). Data were expressed as the mean ± standard deviation. One-way analysis of variance with least square difference test was used to analyze difference. A P value <0.05 was considered to represent statistical significance.
Results
Erastin induces ferroptosis, but not apoptosis, and inhibits proliferation, migration, and invasion in CRC cells
To identify the optimal treatment conditions for erastin, HCT116 cells were treated with different concentrations of erastin at different timepoints. By evaluating the indicators of cell ferroptosis, including Fe2+, MDA, GSH, and GPX, 20 µmol/L Erastin for 48 h was revealed to considerably induce ferroptosis in HCT116 cells compared to other concentrations and times (Figure 1), indicating that this concentration and time were the optimal conditions for erastin treatment of HCT116 cells.

Subsequently, proliferation, migration, and invasion in erastin-treated HCT116 cells were assessed after treatment with erastin at 20 µmol/L for 48 h. Ferrostatin-1, a ferroptosis inhibitor, was used as a negative control. The results of the CCK8 assay showed that erastin greatly inhibited HCT116 cell proliferation compared to the control and ferrostatin-1 treatment (Figure 2A). The Transwell assay also revealed that the migratory and invasion abilities of HCT116 cells were suppressed after erastin treatment compared to the control and ferrostatin-1 treatment (Figure 2B,C).
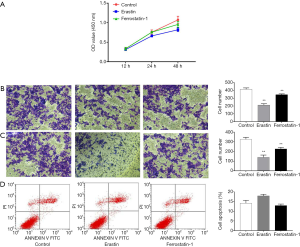
To explore the influence of erastin on HCT116 cell apoptosis, flow cytometry was performed to measure the apoptosis rate. No significant difference was observed in the apoptosis rate between the cells treated with erastin, the control, or ferrostatin-1, suggesting that erastin has little effect on apoptosis in HCT116cells (Figure 2D). Collectively, these results demonstrated that erastin induces ferroptosis but not apoptosis in HCT116 cells, and inhibits their proliferation, migration, and invasion.
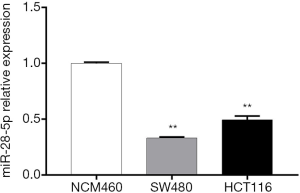
miR-28-5p is downregulated in CRC cells
RT-PCR was used to examine miR-28-5p expression in two colon cancer cell lines, HCT116 and SW480, and one normal human colon line, NCM460. The expression levels of miR-28-5p were significantly lower in both HCT116 and SW480 cells than in NCM460 cells (P<0.01) (Figure 3), which confirmed that miR-28-5p was downregulated in colon cancer cells. In addition, the expression of miR-28-5p was found to be lower in HCT116 cells than in SW480 cells; therefore, HCT116 cells were selected for subsequent experiments.
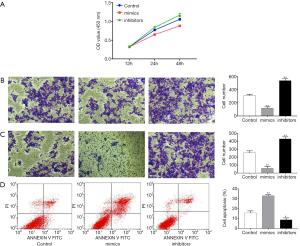
Overexpression of miR-28-5p inhibits proliferation, migration, and invasion in CRC cells
The effect of miR-28-5p in HCT116 cells, was investigated by transfecting HCT116 cells with miR-28-5p mimics or inhibitors and assessing proliferation, migration, invasion, and apoptosis. As shown in Figure 4A, cell proliferation was significantly inhibited by the transfection of miR-28-5p mimics, whereas proliferation was increased by miR-28-5p inhibitors. The Transwell assay revealed that migration and invasion were suppressed after miR-28-5p mimics were transfected but enhanced after miR-28-5p inhibitors were transfected (Figure 4B,C). Flow cytometry also showed that the apoptosis rate was elevated in HCT116 cells after they were transfected with miR-28-5p mimics but decreased after the cells were transfected with miR-28-5p inhibitors (Figure 4D).
Overexpression of miR-28-5p promotes erastin-induced ferroptosis in CRC cells
Erastin treatment elevated the levels of miR-28-5p expression in HCT116 cells, which indicated that miR-28-5p is increased in HCT116 cells undergoing ferroptosis (Figure 5A).
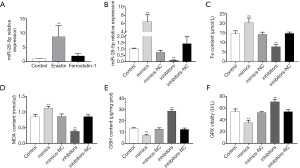
To explore the role of miR-28-5p in erastin-induced ferroptosis in HCT116 cells, miR-28-5p mimics or inhibitors were transfected into HCT116 cells for 24 h, and the cells were then treated with erastin. RT-PCR was used to assessed miR-28-5p expression. After the transfection of miR-28-5p mimics, miR-28-5p expression was significantly increased (P<0.01), whereas miR-28-5p expression was decreased after miR-28-5p inhibitors were transfected (Figure 5B).
The Fe, MDA, GSH, and GPX content in the HCT116 cells were measured after the transfection of mimics or inhibitors and treatment with erastin for 48 h. GSH and GPX were significantly decreased, while Fe and MDA were increased after the transfection of miR-28-5p mimics; however, transfection of miR-28-5p inhibitors did not significantly affect the content of MDA, GSH, or and GPX in erastin-treated cells (Figure 5C,D,E,F). These results showed that miR-28-5p overexpression could promote ferroptosis in HCT116 cells.
miR-28-5p decreases N4BP1 levels after Erastin treatment in CRC cells
A previous study showed that miR-28-5p decreases the levels of N4BP1 in two ovarian cancer cell lines, indicating that N4BP1 is a target gene of miR-28-5p (10). However, whether miR-28-5p regulates N4BP1 in CRC has not been established. To evaluate regulation of N4BP1 by miR-28-5p in erastin-treated CRC cells, we first measured the expression of N4BP1 in NCM460, SW480, and HCT116 cells using RT-PCR. N4BP1 was significantly increased in SW480 and HCT116 cells compared to NCM460 cells (Figure 6A), suggesting that the expression of N4BP1 was also increased in colon cancer cells. Next, we assessed the levels of N4BP1 in erastin-treated HCT116 cells; N4BP1 expression was decreased after treatment with erastin (Figure 6B).
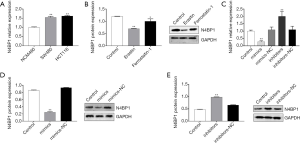
To determine the association between miR-28-5p and N4BP1 in HCT116 cells, we transfected miR-28-5p mimics and inhibitors into HCT116 cells before erastin treatment with, and the expression of N4BP1 was tested by RT-PCR and Western blotting. Both the mRNA and protein expression of N4BP1 were significantly decreased in cells transfected with miR-28-5p mimics compared to those transfected with inhibitors (P<0.05). These results demonstrated that miR-28-5p downregulates N4BP1 expression in HCT116 cells treated with erastin (Figure 6C,D,E).
Discussion
The induction of regulated non-apoptotic cell death has been put forward as an alternative approach for the elimination of drug-resistant cancer cells (12). Ferroptosis, a newly identified type of regulated cell death, occurs when iron-based lipid reactive oxygen species (ROS) accumulates (13). Because of genetic alterations and aberrant proliferation, migration, and invasion, the levels of ROS-related oxidative stress displayed by cancer cells are consistently high. Therefore, agents affecting ROS metabolism can influence tumor cell growth (14). Erastin has been shown to eliminate rat sarcoma viral oncogene-mutated cancer cells via the induction of ferroptosis (15,16). However, the molecular mechanisms of ferroptosis regulation have not been fully explored.
In this study, we examined the effect of erastin on colon cancer cells and observed that it induces ferroptosis in a dose- and time- dependent manner. However, as in other cancer cells, erastin failed to induce apoptosis at any time point or dose. More importantly, we observed that proliferation, migration, and invasion are decreased in erastin-treated cells, suggesting that the induction of ferroptosis might serve as a treatment strategy for colon cancer.
Although previous studies have described some molecular mechanisms of ferroptosis in cancer, few have reported the regulatory role of miRNAs in ferroptosis in cancer cells. Recently, Niu et al. (17) reported that the miR-103a-3p/glutaminase 2 (GLS2) axis was involved in the regulation of physcion 8-O-β-glucopyranoside-triggered ferroptosis and suppressed carcinogenesis in vitro and in vivo. Another study showed that miR-17-92 asserts a protective effect over endothelial cells after erastin-induced ferroptosis and promotes acyl-CoA synthetase4 (ACSL4) expression (18). In addition, miR-9 and miR-137 have been shown to regulate ferroptosis in melanoma through their target genes in vivo and in vitro (19,20).
miR-28-5p is an intragenic miRNA with aberrant expression in several cancers. Increased miR-28-5pexpression has been found in ovarian (10), esophageal (21), and cervical cancer (22) and low expression has been observed in hepatocellular carcinoma (23), renal cell carcinoma (24), and CRC (11), suggesting miR-28-5p takes up different roles during carcinogenesis. A high level of miR-28-5p expression in CRC has been shown to be a predictor of short-term relapse in node-negative patients and of poor overall survival in patients with no metastasis (25). Using in vivo and in vitro experiments, Wu et al. observed that miR-28-5p could negatively regulate SSRP1 expression, thereby inhibiting CRC progression (26). However, no study has reported the activity of miR-28-5p in ferroptosis.
To examine the effect of miR-28-5pon the phenotype of CRC cells and its regulation in ferroptosis, we overexpressed and inhibited miR-28-5pin HCT116 cells treated with erastin and observed miR-28-5p overexpression significantly inhibited proliferation, migration, and invasion and also promoted ferroptosis, indicating that overexpression of miR-28-5p might prove useful in treating CRC. To explore the molecular mechanism behind the regulatory involvement of miR-28-5p in ferroptosis, we identified N4BP1 as a target of miR-28-5pin CRC and showed that N4BP1 was decreased in erastin-treated HCT116 cells. Moreover, N4BP1 expression was induced by miR-28-5p suppression and was downregulated by miR-28-5p overexpression. Overall, our findings demonstrate the important regulatory role miR-28-5p plays in ferroptosis in CRC cells via the regulation of N4BP1.
Limitation
Only HCT116 cell line is selected in the study of tumor cell function, we will consider using a variety of cells to test our conclusions in the future. Luciferase reporter gene to analyze the direct interaction between miR-28-5p and N4BP1 is in our next research plan.
Conclusions
The present study discovered that miR-28-5p promoted ferroptosis in CRC cells by targeting N4BP1. These findings highlight the significance of miRNA in ferroptosis and present miR-28-5p as a key regulator of ferroptosis in colon cancer.
Acknowledgments
Funding: This study was partially supported by research funding from
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1809
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1809). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med 2019;7:609. [Crossref] [PubMed]
- Kumari A, Folk WP, Sakamuro D. The Dual Roles of MYC in Genomic Instability and Cancer Chemoresistance. Genes (Basel) 2017; [Crossref] [PubMed]
- Riddell IA. Cisplatin and Oxaliplatin: Our Current Understanding of Their Actions. Met Ions Life Sci 2018; [Crossref] [PubMed]
- Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017;171:273-85. [Crossref] [PubMed]
- Nguyen THP, Mahalakshmi B, Velmurugan BK. Functional role of ferroptosis on cancers, activation and deactivation by various therapeutic candidates-an update. Chem Biol Interact 2020;317:108930. [Crossref] [PubMed]
- Wang H, Liu C, Zhao Y, et al. Mitochondria regulation in ferroptosis. Eur J Cell Biol 2020;99:151058. [PubMed]
- Shi ZZ, Fan ZW, Chen YX, et al. Ferroptosis in Carcinoma: Regulatory Mechanisms and New Method for Cancer Therapy. Onco Targets Ther 2019;12:11291-304. [Crossref] [PubMed]
- Sui X, Zhang R, Liu S, et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front Pharmacol 2018;9:1371. [Crossref] [PubMed]
- Wang H, Huang C, Yao X. The functions of long non-coding RNAs in colorectal cancer. Transl Cancer Res 2019;8:2192-204. [Crossref]
- Xu J, Jiang N, Shi H, et al. miR-28-5p promotes the development and progression of ovarian cancer through inhibition of N4BP1. Int J Oncol 2017; [Epub ahead of print]. [Crossref]
- Almeida MI, Nicoloso MS, Zeng L, et al. Strand-specific miR-28-5p and miR-28-3p have distinct effects in colorectal cancer cells. Gastroenterology 2012;142:886-96.e9. [Crossref] [PubMed]
- Yang WS. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014;156:317-31. [Crossref] [PubMed]
- Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ 2016;23:369-79. [Crossref] [PubMed]
- Cramer SL, Saha A, Liu J, et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med 2017;23:120-7. [Crossref] [PubMed]
- Dolma S, Lessnick SL, Hahn WC, et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003;3:285-96. [Crossref] [PubMed]
- Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 2008;15:234-45. [Crossref] [PubMed]
- Niu Y, Zhang J, Tong Y, et al. Physcion 8-O-beta-glucopyranoside induced ferroptosis via regulating miR-103a-3p/GLS2 axis in gastric cancer. Life Sci 2019;237:116893. [Crossref] [PubMed]
- Xiao FJ, Zhang D, Wu Y, et al. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem Biophys Res Commun 2019;515:448-54. [Crossref] [PubMed]
- Luo M, Wu L, Zhang K, et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ 2018;25:1457-72. [Crossref] [PubMed]
- Zhang K, Wu L, Zhang P, et al. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol Carcinog 2018;57:1566-76. [Crossref] [PubMed]
- Chen L, Jin Y, Wang L, et al. Identification of reference genes and miRNAs for qRT-PCR in human esophageal squamous cell carcinoma. Med Oncol 2017;34:2. [Crossref] [PubMed]
- Wilting SM, Snijders PJ, Verlaat W, et al. Altered microRNA expression associated with chromosomal changes contributes to cervical carcinogenesis. Oncogene 2013;32:106-16. [Crossref] [PubMed]
- Shi X, Teng F. Down-regulated miR-28-5p in human hepatocellular carcinoma correlated with tumor proliferation and migration by targeting insulin-like growth factor-1 (IGF-1). Mol Cell Biochem 2015;408:283-93. [Crossref] [PubMed]
- Wang C, Wu C, Yang Q, et al. miR-28-5p acts as a tumor suppressor in renal cell carcinoma for multiple antitumor effects by targeting RAP1B. Oncotarget 2016;7:73888-902. [PubMed]
- Tsiakanikas P, Kontos CK, Kerimis D, et al. High microRNA-28-5p expression in colorectal adenocarcinoma predicts short-term relapse of node-negative patients and poor overall survival of patients with non-metastatic disease. Clin Chem Lab Med 2018;56:990-1000. [Crossref] [PubMed]
- Wu W, He K, Guo Q, et al. SSRP1 promotes colorectal cancer progression and is negatively regulated by miR-28-5p. J Cell Mol Med 2019;23:3118-29. [Crossref] [PubMed]