
The value of intra-procedural transcatheter intraarterial contrast-enhanced ultrasonography (IA-CEUS) in predicting the short-term efficacy of conventional transarterial chemoembolization (cTACE)
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, its global incidence ranks fifth in cancers, and its mortality rate ranks third (1). Conventional transarterial chemoembolization (cTACE) is the standard treatment for HCC patients in Barcelona Clinic Liver Cancer (BCLC) stage B and can significantly improve the survival rate of patients (1-3). Although the overall survival time remains the best evaluation index for cTACE in clinical trials, image biomarkers are often used to replace the overall survival time to predict the short-term and long-term efficacy of cTACE (4-7). Therefore, it is of great clinical significance to select appropriate image biomarkers for the early and accurate evaluation of the embolization effect of cTACE for selecting an appropriate follow-up treatment regimen.
Intra-arterial contrast-enhanced ultrasonography (IA-CEUS) is a new imaging technique, which can display blood flow and tissue perfusion via a microbubble contrast agent and then achieve the purpose of real-time display of diseased vessels and tissues (8,9). Previous studies revealed that TACE-assisted IA-CEUS could improve the accuracy and success rate of embolization and the efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE). The results also preliminarily revealed the safety and value of IA-CEUS in cTACE (8,10). Compared with intravenous CEUS, digital subtraction angiography (DSA), multidetector CT (MDCT), cone-beam computed tomography (CBCT), and magnetic resonance imaging (MRI), IA-CEUS can dynamically observe the change of microcirculation of the whole tumor of HCC in real-time, including the changes in blood flow from the artery to the tumor, intratumoral perfusion, and the local drainage area around the tumor. However, the change of tumor microcirculation after TACE is closely related to the success rate of embolization, short-term and long-term curative effects, and recurrence (4,11,12). Therefore, it is of great clinical significance in predicting a curative effect to observe the changes of tumor microcirculation with IA-CEUS immediately after TACE. At present, there remains no literature reporting on the prediction of the short-term curative effect by IA-CEUS immediately after cTACE.
The purpose of this study was, therefore, to evaluate the specific clinical value of IA-CEUS as an imaging biomarker in predicting the short-term curative effect of cTACE through studying the characteristics of the changes in the tumor and drainage areas of HCC in CEUS immediately after cTACE.
Methods
Study subjects
This study was a prospective single-center clinical trial. From August 2018 to March 2019, the patients who were positively diagnosed with HCC and received cTACE in the General Hospital of People’s Liberation Army were selected as the main study subjects. Indications of cTACE: single or multiple surgery-intolerant HCC, Eastern Cooperative Oncology Group (ECOG) scored 0 or 1, the Child-Pugh stage was A or B, and target lesions had not been treated. The Ethics Committee of the General Hospital of People’s Liberation Army approved this study and all patients signed informed consent.
Inclusion and exclusion criteria
Inclusion criteria: (I) HCC was diagnosed according to the diagnostic criteria of clinical guidelines; (II) gray-scale ultrasound could clearly display the lesions of the patient in the supine position; (III) 1 cm ≤ was the maximum diameter of lesions <10 cm; (IV) the target lesions were nodular, which could be clearly displayed on enhanced MRI; (V) IA-CEUS revealed the drainage area of the target lesions: the ring-like high enhancement area around the lesions in the late stage of enhancement.
Exclusion criteria: (I) the lesion range was >70%; (II) patients with cancer thrombus of the portal vein trunk; (III) patients with extensive extrahepatic metastasis; (IV) patients with poor liver function (Child-Pugh C); (V) patients without enhanced MRI data 1–2 months after the operation; (VI) preoperative contrast-enhanced imaging showed that the lesions were necrotic; (VII) the target lesions were treated with local or systemic therapy before the operation; (VIII) preoperative contrast-enhanced imaging showed that there was abnormal perfusion or arteriovenous fistula in the target area; and (IX) the collateral vessels of the target lesions participated in the blood supply.
TACE
Transcatheter interventional therapy for liver cancer adopts cTACE with lipiodol as the carrier of chemotherapeutic drugs. All interventional operations were performed by ZJ Wang, who had 16 years of experience in minimally invasive liver cancer treatment and followed our hospital’s standard operation method. The femoral artery was punctured in the modified Seldinger method, then the routine celiac artery and superior mesenteric artery angiography were performed with a 4-F ordinary catheter (RH, Terumo Corporation, Japan). If necessary, selective hepatic arteriography was also performed. Super selective intubation with a 3F microcatheter (Progreat, Terumo Corporation, Japan) in the tumor blood supply artery was performed for chemoembolization. Intraoperative medication: 3–4 chemotherapeutic drugs including Epirubicin (30–50 mg), Cisplatin (40–60 mg) or Oxaliplatin (100–150 mg), Mitomycin (10–14 mg), 5-Fluorouracil (5-FU) (500–750 mg), and Leucovorin (200–300 mg), the powders of which were mixed with lipiodol at a proportion of 1:1 to form an emulsion and liquid chemotherapeutic drugs were used for direct target vessel perfusion via a microcatheter. After the lipiodol emulsion perfusion, the combination of polyvinyl alcohol (PVA) and gelatin sponge particles with the size of 100–300 or 300–500 µm were used for embolization. The retention of a contrast reagent in the tumor supply artery was used as the endpoint for the embolization.
IA-CEUS technology
In the present study, a Siemens S2000 HELX ultrasound machine was used. The probes for both the conventional ultrasound and CEUS were 6C1-HD. The probe frequency was 3–5 MHz in the gray-scale imaging mode. The contrast pulsing sequence (CPS) was also used in CEUS. The probe frequency was set at 1.5–2.0 MHz in the CEUS imaging. The mechanical index of CEUS was 0.05–0.07. A sulfur hexafluoride microbubble for injection (SonoVue, Bracco, Italy) was used as an ultrasound contrast agent. An ultrasound contrast agent was configured as 5 mL of the suspension according to the instructions and 0.2 mL of the contrast agent suspension was used and diluted to 10 mL (1:50) with normal saline. IA-CEUS adopted the bolus injection method and according to the size of the lesions, the dose of contrast agent was 2.0–4.0 mL/time and the injection rate was 1 m/s. Before the operation, for patients who met the inclusion criteria, the target lesions were determined by conventional ultrasound and the images were stored, the CEUS mode was started and the ultrasound contrast agent was injected through the proper hepatic artery and the timing was started. The dynamic CEUS images were stored continuously for at least 3 minutes. Immediately after the cTACE, the target lesions were confirmed again by conventional ultrasound, then the CEUS mode was started and the contrast agent was injected via the proper hepatic artery and the timer was started. The dynamic CEUS images were stored continuously for at least 3 minutes. The CEUS observation indexes of the hepatic artery after the interventional operation of the target lesion were as follows: (I) the maximum cross-sectional area ratio of the internal enhancement area of the tumor: the ratio of the maximum cross-sectional area of the internal enhancement area of the tumor after the operation compared to that before the operation; (II) the enhanced peak intensity of the drainage area after the operation; (III) the thickness of the drainage area (cm) after the operation: the thickness of the drainage area was measured at the thickest site.
The ultrasound image interpretation method after the interventional operation
Image interpretation was performed independently by two physicians with over 8 years of experience in the abdominal CEUS. Both physicians were unaware of the general condition, laboratory tests, and the patients’ additional image findings. According to the design of this study, the images of all subjects were analyzed and recorded by two physicians. If the image analysis results from the two physicians were inconsistent, after discussion, they reached an agreement on the final result and recorded it.
The evaluation of the curative effect
At 1–2 months after the operation, enhanced MRI and routine blood test, liver and kidney function tests, tumor markers [alpha-fetal protein (AFP), carbohydrate antigen 199 (CA199), and carcinoembryonic antigen (CEA)] tests were performed. If the tumor was well controlled, the patient was followed up every 2–3 months. All imaging data were reviewed by radiologists with over 8 years of experience. The curative effect of the interventional therapy was evaluated by modified response evaluation criteria via the solid tumor (mRECIST) method: (I) Complete remission (CR): there is no image enhancement of lesions in or around the tumor. (II) Partial remission (PR): the diameter of measurable lesions is reduced by ≥30% in the arterial enhancement phase. (III) Stable disease (SD): the lesions between PR and progressive disease (PD) indicated SD. (IV) PD: the diameter of the measurable lesions increased by ≥20% in the arterial enhancement phase.
Statistical analysis
Data were analyzed using statistical software SPSS 24.0. Measurement data were expressed as mean ± standard deviation (mean ± SD). Count data were expressed as a percentage (%). The correlation between the ratio of maximum cross-sectional tumor area before the operation to that post-operation, postoperative drainage area peak intensity, and thickness with the curative effect was analyzed using the Spearman’s correlation analysis. According to the standard, the rho value of the correlation coefficient was divided into five grades: 0–0.2, 0.2–0.4, 0.4–0.6, 0.6–0.8, and 0.8–1, which were defined as poor, fair, moderate, good, and excellent, respectively. P<0.05 was considered statistically significant.
Results
General conditions
From August 2018 to March 2019, 39 consecutive patients were enrolled in this study. Among these patients, 34 patients were male, and five patients were female, and these patients had a total of 51 lesions. The average age of these patients was 60.3±11.2 [32–78] years old. The basic indices are shown in Table 1. The average maximal diameter of the target lesion was 3.72±2.43 (1.2–10.0) cm. The ultrasonic characteristics of the lesions are shown in Table 2.
Table 1
| Parameter | Value |
|---|---|
| No. of patients | 39 |
| No. of tumors evaluated | 51 |
| Patient age* (years) | 60.3±11.2 [32–78] |
| Sex | |
| Male | 35 |
| Female | 4 |
| Etiology | |
| Hepatitis B virus | 34 |
| Hepatitis C virus | 4 |
| Hepatitis B & C virus | 1 |
| Cirrhosis | |
| Present | 29 |
| Absent | 10 |
| Child-Pugh stage | |
| A | 15 |
| B | 24 |
| Barcelona Clinic Liver Cancer stage | |
| A | 4 |
| B | 24 |
| C | 11 |
| ECOG | |
| 0 | 29 |
| 1 | 10 |
*, data are expressed as means ± standard deviations [range]. ECOG, Eastern Cooperative Oncology Group.
Table 2
| Features | Value |
|---|---|
| Segment | |
| S1 | 0 |
| S2 | 4 |
| S3 | 7 |
| S4 | 2 |
| S5 | 14 |
| S6 | 9 |
| S7 | 8 |
| S8 | 7 |
| Average tumor maximum size* (cm) | 3.72±2.43 (1.2–10.0) |
| Echogenicity | |
| Hypoechoic | 24 |
| Isoechoic | 10 |
| Hyperechoic | 17 |
| CDFI | |
| Present | 28 |
| Absent | 23 |
*, data are expressed as means ± standard deviations (range). CDFI, color Doppler flow imaging.
The evaluation of the postoperative effect of patients with cTACE
At 1–2 months after cTACE, the curative effect of the target lesion was evaluated using mRECIST. The results revealed that, of these 51 lesions, the evaluation results of the curative effect were in CR in 22 lesions (43.1%), in PR in 14 lesions (27.5%), SD in 10 lesions (19.6%), and PD in 5 lesions.
The correlation between the characteristics of IA-CEUS and the curative effect
The characteristic values of the maximum cross-sectional area ratio, the postoperative peak intensity, and the thickness of the drainage area are shown in Table 3. In the CR group, the IA-CEUS immediately after the operation revealed that the drainage areas of the lesions disappeared (Figure 1); in the PR, SD, and PD groups, the IA-CEUS immediately after the operation revealed that there remained different degrees of enhancement in the drainage areas of the lesions (Figure 2). Statistical analysis results revealed that the three parameters of the intra-tumor enhancement area ratio, the postoperative peak intensity, and the drainage area thickness were significantly correlated with the curative effect [Spearman rho =0.513, P=0.000 (<0.01); rho =0.671, P=0.000 (<0.01); rho =0.704, P=0.000 (<0.01), Table 3]. Compared with the perfusion index in the tumor, the postoperative peak intensity and the drainage area thickness were more closely correlated with the short-term effect of the cTACE.
Table 3
| Items | CR | PR | SD | PD | r | P |
|---|---|---|---|---|---|---|
| Maximum cross-sectional area ratio | 0.169±0.287 | 0.434±0.274 | 0.390±0.252 | 0.825±0.349 | 0.513 | 0.000 |
| Postoperative peak intensity (dB) | 0.617±2.451 | 1.638±2.294 | 8.917±5.302 | 12.594±3.244 | 0.671 | 0.000 |
| Thickness of the drainage area (cm) | 0.019±0.100 | 0.328±0.447 | 0.611±0.380 | 0.944±0.518 | 0.704 | 0.000 |
CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
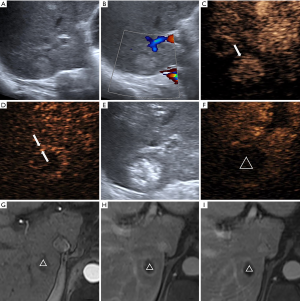
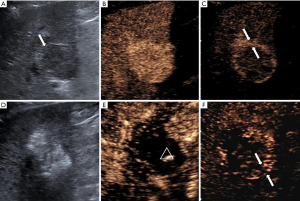
Discussion
A total of 39 patients with 51 lesions were included in this present study. The tumor response in patients after cTACE was assessed and the results revealed that CR, PR, SD and PD were achieved in 22 lesions (43.1%), 14 lesions (27.5%), 10 lesions (19.6%), and 5 lesions (9.8%), respectively. The maximum cross-sectional area ratio of intratumoral perfusion was moderately correlated with the tumor response on the contrast-enhanced MRI (CE-MRI). The peak value of coronary enhancement and lesions was highly correlated with the tumor response on the CE-MRI.
Although tumor pathology is the gold standard for cTACE treatment, it is difficult to implement in clinical practice. There is a clear correlation between the imaging findings and pathology in the near future (1–2 months) after cTACE. Clinically, the interventional effect assessed by mRECIST is used to replace pathological examination, but the disadvantage is that the waiting time is long and it is impossible to determine the curative effect and take further effective measures in time. The endpoint of embolization or technical success rate in interventional tumor surgery is closely correlated to its short-term and long-term effects (4,13). The early and accurate determination of embolization is of great significance to take effective measures in time and improve the short-term and long-term efficacy (14,15). Excessive embolization will lead to liver failure, tumor necrosis, and tumor hypoxia and then causes the production of tumor vascular endothelial growth factor (VEGF) and leads to tumor recurrence. Insufficient embolization will make chemotherapeutic drugs difficult to absorb for a long time, play an anti-tumor role, and lead to the recanalization of blood vessels (13,16).
The retention of contrast agent in the local artery and intratumoral perfusion after arterial embolization are the main technical indices for imaging prediction of short-term and long-term effects. It is the most common method to predict the long-term effect of cTACE by observing the retention of contrast agent under fluoroscopy during an interventional operation (that is, DSA), the disadvantage is that it does not take into account the changes of tumor perfusion and drug distribution; due to the limitations of imaging technology, results are subjective and variable (4,17,18). DSA combined with CBCT and MRI to evaluate the local tumor perfusion changes post-cTACE to predict short-term efficacy has become the main technical means at present (11,18). Local tumor blood flow assessed by CBCT or MRI combined with transcatheter arterial catheterization can predict the short-term and long-term effects of cTACE (6,13,18). In the present study, for the first time, IA-CEUS technology was used to observe the changes of intratumoral perfusion in the tumor microenvironment during cTACE to evaluate its predictive value for the efficacy of cTACE. The results revealed that there was a significant correlation between the change ratio of the maximum cross-sectional area of the enhanced area in the tumor and the curative effect. The results confirmed the value of IA-CEUS in predicting the short-term effect of cTACE via intratumoral perfusion. Compared with DSA and CBCT, IA-CEUS has the advantages of simple operation, real-time dynamic observation, the absence of radiation, and contrast agent nephrotoxicity. Compared with an MRI, ultrasound equipment is mobile and able to be implemented in most medical centers.
Compared with the above-mentioned DSA, CBCT, and MR imaging techniques, IA-CEUS can also dynamically observe the real-time change in the microcirculation of the entire tumor post-cTACE, observe the blood flow perfusion of the artery into the tumor and intratumoral perfusion, and also observe changes in the local drainage area around the tumor. The HCC drainage area is dominated by the drainage veins around the tumor, which is the intermediate link between the tumor and the peripheral portal veins. The vascular characteristics of the drainage area during cTACE are closely correlated to tumor grade and curative effect and is an important circulatory pathway for intrahepatic and extrahepatic metastasis of a tumor (12). When evaluating the curative effect of the cTACE on the HCC, we should not only pay attention to the characteristics of intra-tumor enhancement but also to the characteristics of the tumor drainage area enhancement. Kitao et al. (19) first reported the presence of the HCC drainage area via CT hepatic arteriography, which was confirmed by pathology as drainage veins. Miyayama et al. (20) first used transcatheter hepatic artery catheterization combined with dual-phase CBCT to observe the ability of an HCC corona enhancement and describe its characteristics. Müller et al. (12) used dual-phase CBCT to observe the characteristics of the drainage area before cTACE and the result revealed that the image characteristics of CBCT in the drainage area were related to the curative effect. The drainage area with diffuse and flaky characteristics decreases the possibility of CR post-cTACE, so other alternative therapies such as cTACE or ablation should be considered. In the present study, the advantage of IA-CEUS technology that can be used to dynamically observe the drainage area of target lesions in real-time was used and for the first time, IA-CEUS was used to quantitatively observe the peak intensity and thickness of the drainage area post-cTACE in different curative effect groups and the correlation between them, and the curative effect was evaluated. A previous study revealed that the two parameters of peak intensity and thickness of the drainage area post-cTACE were significantly correlated with the curative effect, suggesting that after cTACE, the characteristics of the tumor drainage area can predict the effect of cTACE. Particularly, compared with the simple intratumoral perfusion index, the drainage area is more closely correlated with the curative effect on the tumor. The reason may be that the drainage area is located in the peripheral area, which is the area where the ultrasound contrast agents are relatively concentrated and observing the intratumoral perfusion changes by CEUS is mainly based on observing a certain section of the tumor and the influence of lipiodol deposition. In clinical practice, IA-CEUS should not only regard the non-enhancement of intratumoral perfusion as the endpoint or objective imaging basis for successful embolization but also regard the non-enhancement of all drainage areas as the endpoint or objective imaging basis for successful embolization.
This study still has the following limitations: (I) this study is a single-center clinical trial, the included sample size was small, multi-center clinical trials with a larger sample size is still required. (II) The subjectivity and experience of the CEUS operator do affect the reproducibility of the technology to some extent. (III) For certain parts, such as deep lesions, an ultrasound is not able to clearly show the lesions, therefore, with the development of 3D ultrasonic imaging technology, further evaluation is still needed. (IV) There was no comparison between the prediction of IA-CEUS with MRI, CBCT, and MDCT on the curative effect, so, further research is still needed. (V) The TACE technique described uses different embolic materials which may have a different impact on the ultrasound assessment. Therefore, another study performing separate statistical assessments according to the different embolic agents is necessary in the future.
Conclusions
Intra-procedural IA-CEUS may predict short-term tumor response in cTACE of HCC. The feature of corona enhancement immediately post-cTACE also showed a more accurate prediction when compared with the feature of intratumor infusion (rho =0.671).
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted with approval from the Ethics Committee of the First Medical Centre, Chinese PLA General Hospital. All patients signed informed consent. This study was conducted in accordance with the declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Jing CY, Fu YP, Zheng SS, et al. Prognostic nomogram for patients with hepatocellular carcinoma underwent adjuvant transarterial chemoembolization following curative resection. Medicine (Baltimore) 2017;96:e6140. [Crossref] [PubMed]
- Liao M, Zhu Z, Wang H, et al. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol 2017;52:624-34. [Crossref] [PubMed]
- Pavlus J, Sandow T, Cohen E, et al. 3:27 PM Abstract No. 134 Is smaller better for hepatocellular carcinoma? Evaluation of DEB-TACE bead size and cTACE in 142 explanted tumors. J Vasc Interv Radiol 2018;29:S60-1. [Crossref]
- Zori AG, Ismael MN, Limaye AR, et al. Transarterial Radioembolization as Bridge Therapy for Patients with Early Stage Hepatocellular Carcinoma Undergoing Liver Transplantation and Universiy of Florida Experience. Gastroenterology 2017;152:S1177. [Crossref]
- Loffroy R, Lin M, Yenokyan G, et al. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology 2013;266:636-48. [Crossref] [PubMed]
- Sahu S, Schernthaner R, Ardon R, et al. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treated with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology 2017;283:883-94. [Crossref] [PubMed]
- Lekht I, Nayyar M, Luu B, et al. Intra-arterial contrast-enhanced ultrasound (IA CEUS) for localization of hepatocellular carcinoma (HCC) supply during transarterial chemoembolization (TACE): a case series. Abdom Radiol (NY) 2017;42:1400-7. [Crossref] [PubMed]
- Nzekwu E, Mirakhur A, Lee A, et al. Intra-Arterial and Intravenous Contrast-Enhanced Ultrasonography in Prostate Artery Embolization: A Case Series. J Vasc Interv Radiol 2018;29:1399-402. [Crossref] [PubMed]
- Shiozawa K, Watanabe M, Ikehara T, et al. Efficacy of intra-arterial contrast-enhanced ultrasonography during transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. World J Hepatol 2018;10:95-104. [Crossref] [PubMed]
- Wang D, Gaba RC, Jin B, et al. Intraprocedural transcatheter intra-arterial perfusion MRI as a predictor of tumor response to chemoembolization for hepatocellular carcinoma. Acad Radiol 2011;18:828-36. [Crossref] [PubMed]
- Müller K, Datta S, Gehrisch S, et al. The Role of Dual-Phase Cone-Beam CT in Predicting Short-Term Response after Transarterial Chemoembolization for Hepatocellular Carcinoma. J Vasc Interv Radiol 2017;28:238-45. [Crossref] [PubMed]
- Wang Z, Chen R, Duran R, et al. Intraprocedural 3D Quantification of Lipiodol Deposition on Cone-Beam CT Predicts Tumor Response After Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2015;38:1548-56. [Crossref] [PubMed]
- Kim HY, Park JW, Joo J, et al. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol 2012;27:1051-6. [Crossref] [PubMed]
- Yamanaka K, Hatano E, Kitamura K, et al. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. J Gastroenterol 2012;47:343-6. [Crossref] [PubMed]
- Duran R, Mirpour S, Pekurovsky V, et al. Preclinical Benefit of Hypoxia-Activated Intra-arterial Therapy with Evofosfamide in Liver Cancer. Clin Cancer Res 2017;23:536-48. [Crossref] [PubMed]
- de Korompay N, Alshammari M, Klass D, et al. Intraprocedural Parenchymal Blood Volume Is a Predictor of Treatment Response for Chemoembolization in Hepatocellular Carcinoma: Results of a Prospective Study. J Vasc Interv Radiol 2018;29:928-35. [Crossref] [PubMed]
- Kim KA, Choi SY, Kim MU, et al. The Efficacy of Cone-Beam CT-Based Liver Perfusion Mapping to Predict Initial Response of Hepatocellular Carcinoma to Transarterial Chemoembolization. J Vasc Interv Radiol 2019;30:358-69. [Crossref] [PubMed]
- Kitao A, Matsui O, Yoneda N, et al. Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 2012;265:780-9. [Crossref] [PubMed]
- Miyayama S, Yamashiro M, Okuda M, et al. Detection of corona enhancement of hypervascular hepatocellular carcinoma by C-arm dual-phase cone-beam CT during hepatic arteriography. Cardiovasc Intervent Radiol 2011;34:81-6. [Crossref] [PubMed]


