
The efficacy of afatinib in patients with HER2 mutant non-small cell lung cancer: a meta-analysis
Introduction
Targeted therapy has been well-documented to improve the outcomes of non-small cell lung cancer (NSCLC) patients carrying activating driver mutations, such as epidermal growth factor receptor (EGFR), translocations in the receptor tyrosine kinase (ALK), c-Ros proto-oncogene 1 receptor tyrosine kinase (ROS1) (1-3). Erb-b2 receptor tyrosine kinase 2 (ErbB2/HER2) mutations have been found in approximately 2–4% of NSCLC cases and are known as carcinogenic mutations with higher prevalence in women and never-smokers (2,4-6). There are increasing evidence supporting the use of anti-HER2 agents in HER2 mutant NSCLC (6-8).
Afatinib is an anilino-quinazoline which can irreversibly bind to EGFR and HER2, potently suppressing the kinase activity of wild-type and activated EGFR and HER2 mutants (9). As a member of irreversible ErbB family inhibitor, afatinib has been investigated to exhibit clinical activity in patients with HER2-mutant NSCLC (9-12). However, the efficacy varied and thus the effectiveness remains uncertain (13).
Here, we performed an exhaustive literature search, capturing all available data regarding the activity of afatinib in HER2-mutant NSCLC, and reanalyzed the efficacy and toxicity of afatinib, aiming to provide novel insight into the association between afatinib and HER2-mutant NSCLC.
Methods
Search strategy
We conducted a systematic literature search for published articles about afatinib treatment for NSCLC patients with HER2-mutations up to April 2019 in the PubMed, EMBASE and Cochrane Library databases, using the following terms: [“afatinib” or “(2E)-N-(4-(3-Chloro-4-fluoroanilino)-7-(((3S)-oxolan-3-yl)oxy)quinoxazolin-6-yl)-4(dimethylamino)but-2-enamide” or “BIBW 2992 MA2” or “Afatinib Maleate” or “BIBW 2992” or “Gilotrif” or “Afatinib Dimaleate”] and (“Carcinoma, Non-Small-Cell Lung” or “Lung Carcinoma, Non-Small-Cell” or “Non-Small-Cell Lung Carcinomas” or “Non-Small Cell Lung Cancer”) and (“Erb-b2 Receptor Tyrosine Kinases” or “ ErbB-2 Receptor” or “Oncogene Protein HER-2” or “Tyrosine Kinase-type Cell Surface Receptor HER2” or “c-ErbB-2, Proto-oncogene” or “HER-2 Proto-Oncogene Protein” or “erbB-2 Receptor Protein-Tyrosine Kinase” or “Proto Oncogene Proteins c erbB-2”). To ensure a complete acquisition of the relevant literature, we performed independent supplemental manual search on the reference lists of retrieved articles. To avoid a local literature bias, the search was diffusely designed without region restrictions, but only studies published in English were included.
Inclusion and exclusion criteria
The full text of the retrieved articles were reviewed to determine whether the topic and information presented were suitable. Study selection was performed in accordance with the following inclusion criteria: (I) the participants received afatinib monotherapy; (II) the manuscript had adequate descriptions of the diagnostic criteria for NSCLC patients with HER2 mutations and exactable outcomes. The exclusion criteria were as follows: (I) the publication type was a review, letter, abstract or comment; (II) the study was not designed as a case-control or cohort study. Two investigators (Jie Zhao and Hui Shen) independently completed the literature retrieval and the discrepancies were resolved by reaching a consensus or using input from a third investigator (the corresponding author), if necessary.
Data extraction
Data were independently collected in duplicate by two authors (Jie Zhao and Hui Shen) using a standard protocol to ensure data accuracy. The following information were extracted from each selected study: (I) the participants’ features; (II) the intervention and time that the participants were disposed; (III) the medical history of the participants; (IV) efficacy outcomes of interest such as objective response rate (ORR) and disease control rate (DCR); (V) adverse events and subjective feelings.
Statistical analysis
Statistical analyses were performed using R studio (version 1.2.5033). Publication bias was evaluated using the Egger test (<0.05 was considered to indicate publication bias) and the sensitivity analyses (omitting a single study). As the quantity of studies was small, the requirements for a funnel plot were not met. Heterogeneity amongst studies was evaluated with Cochran’s Q and I2 tests. The calculation of the pooled summary statistic and 95% confidence intervals (CIs) were estimated using a random-effect model when between-study heterogeneity was moderate or high. The fixed-effect model was used when between-study heterogeneity was low. All P values were two-sided and those <0.05 were considered significant.
Results
Study characteristics
The literature search identified 63 articles potentially met our requirements. After reviewing, 22 were excluded for not relevant, 24 were excluded for the inclusion of non-NSCLC treatment focus, 7 were excluded for inadequate descriptions of the diagnostic criteria and 2 were excluded due to combined afatinib with other anti-neoplastic medications. The detailed study selection process was depicted in Figure 1. Eventually, 8 studies (1,6,13-18), comprising a total of 95 patients were enrolled in the analysis (Table 1). Patients were ranged from 33 to 93 years old.
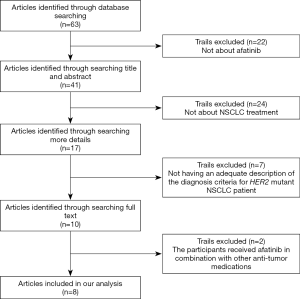
Table 1
| Peters (1) | Mazières (6) | Dziadziuszko (13) | Lai (14) | Costa (15) | Ou (16) | Al-Obeidi (17) | Liu (18) | |
|---|---|---|---|---|---|---|---|---|
| Trait | Retrospective | Retrospective | Prospective | Retrospective | Retrospective | Prospective | Retrospective | Retrospective |
| Location | 9 locationsa | 6 locationsb | Europe | 3 locationsc | Israel | America | Na | China |
| Age (year) | 39–93 | NA | 39–82 | 40–84 | 64–71 | 33–74 | 66–80 | 41–86 |
| Woman (%) | 57 | NA | 69.2 | 41 | 66.7 | 73 | 60 | 63 |
| Smoke history | NA | NA | 5 | 9 | 1 | 4 | 1 | NA |
| Dosage (mg/day) | 40/50 | NA | 40 | 20/30/40 | 280 mg/week | 40 | 20/30/40 | 40 |
| Clinical stage (TNM) | IV | IV | III/IV | IV | IV | NA | IV | IV |
| Line of afatinib treatment | ≥ First | NA | > First | ≥ First | > First | ≥ First | First | NA |
| Inspection method | NA | PCR/NGS | NGS | PCR/NGS | NA | CGP | NA | NGS |
| Number of enrolled patients | 28 | 11 | 13 | 27 | 3 | 15 | 5 | 19 |
| Number of evaluable patients | 16 | 11 | 13 | 23 | 3 | 5 | 5 | 19 |
| A775-G776insYVMA | 10 (36%) | NA | 10 (77%) | 15 (65%) | 1 (33%) | 0 | 3 (60%) | 0 |
| CR | 0 | NA | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 3 | 2 | 1 | 3 | 1 | 2 | 4 | 3 |
| SD | 8 | 5 | 6 | 13 | 1 | 1 | 0 | 10 |
| mPFS (month) | NA | 3.9 | 4.0 | NA | NA | NA | NA | 10.0 (G778_P780dupd); 3.3 (otherse) |
| mOS (month) | NA | NA | 14.0 | 7.0 | NA | NA | NA | 19.7 (G778_P780dupd); 7.0 (otherse) |
| ORR (%) | 18.8 | 18.2 | 7.7 | 13.0 | 33.3 | 40.0 | 80 | 15.8 |
| DCR (%) | 68.8 | 63.7 | 53.8 | 69.6 | 66.7 | 60.0 | 80 | 68.4 |
| Adverse event | NA | NA | NA | NA | NA | |||
| Frequency | 100% | 100% | 100% | |||||
| Grade 0–2 | NA | 13 (100%) | 3 (100%) | |||||
| Grade 3–4 | NA | 5 | 0 | |||||
| Grade 5 | 0 | 1 | 0 |
a, the 6 locations included France, Switzerland, Spain, Italy, Poland, Portugal, and the Netherlands; b, the 9 locations included Switzerland, Spain, Belgium, Germany, The Netherlands, Slovenia, China, Israel, and Argentina; c, the 3 locations included Europe, Australia and North America; d, G778_P780dup means glycine at ERBB2 778 site; e, others means other amino acids at 778 due to insertion. NA, not applicable; CGP, comprehensive genome profiling; NGS, next-generation sequencing; PCR, polymerase chain reaction; CR, complete response; PR, partial response; SD, stable disease; mPFS, median progression-free survival; mOS, median overall survival; ORR, objective response rate; DCR, disease control rate; dup, duplicate.
Results of the meta-analysis
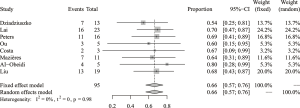
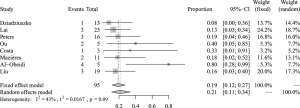
The pooled analysis of DCR concerning afatinib treatment for NSCLC patients with HER2 mutation was based on all 8 analyzed studies (95 evaluable patients) and the results disclosed a pooled DCR of 66% (95% CI: 57–76%) (Figure 2). Between-study heterogeneity was low (I2=0, Cochran’s Q =1.65, P=0.98) and thus a fixed-effect model was preferred. The analysis of ORR was also based on all 8 studies, and the pooled ORR was 21% (95% CI: 11–34%) (Figure 3). Between-study heterogeneity was relatively low (I2=43%, Cochran’s Q =12.28, P=0.09) and thus a random effect model was chosen.
There were also some adverse events happened during the treatment of afatinib (Table S1), most of which were grade 1–2 events including diarrhea, vomiting, abdominal pain, skin rash, paronychia, fatigue, mucositis, and dyspnea. Grade 3–4 events such as dyspnea, epistaxis, pleural effusion, oral mucositis, lung infection, gamma-glutamyl transferase increase, electrolyte abnormalities, urinary tract obstruction, paraplegia, anemia, and febrile neutropenia were uncommon. Of note, it was reported a case suffering from fatal acute renal injury, possibly related to afatinib (13).
Publication bias
The Egger test showed that publication bias did not exist for DCR (P=0.9503) or ORR (P=0.04626). However, the sensitivity analyses indicated that the analysis of DCR and ORR were all stable, with almost all estimates between the lower and upper confident interval limits (Tables 2,3). Therefore, the included studies were considered to be reliable.
Discussion
The present meta-analysis demonstrated that afatinib monotherapy elicited moderate anti-tumor activity in HER2 mutant NSCLC. The pooled ORR was 21% and the pooled DCR was 66%. The patients harboring A775-G776insYVMA mutation, the most common HER2 exon 20 mutation, derived larger clinical benefit from backline afatinib, with longer disease stabilization. The response heterogeneity to afatinib may due to the divergent subtypes of HER2 mutations, even within the subtypes of HER2 exon 20 mutations. HER2 exon 20 G778_P780dup mutation demonstrated longer median progression-free survival (mPFS) and median overall survival (mOS) than other subsets in response to afatinib, albeit not statistically significant, possibly owing to the glycine at HER2 778 site was a primary drug sensitive mutation (10,18). HER2 transmembrane domain (TMD) mutations (HER2 V659E, HER2 G660D), located on exon 17, was reported as a group of emerging actionable oncogenic alterations in NSCLC. TMD mutation changes amino acids at V695 and G660 position to increase the polarity of the cavity itself, thereby stabilizing homo and heterodimers of HER family, resulting in uncontrolled receptor activation. Ou and colleagues reported afatinib was effective for HER2 TMD mutation with an ORR of 40% (2/5), but the limited number of patients makes made it hard to draw a definite conclusion (16).
In NSCLC, HER2 alterations occur in 2–4% of patients, most commonly in adenocarcinoma and never smokers. There are three approaches of treating HER2 alterations, including small molecule TKIs, chemotherapy and anti-HER2 antibody.
TKIs targeted to HER2 mutation have been fully investigated, including afatinib, poziotinib and pyrotinib, lapatinib, neratinib. Our finding here showed HER2 A775-G776insYVMA mutation benefited more from afatinib, while the overall therapeutic effect on other HER2 mutation subtypes was moderate. Recent emerging poziotinib, an agent targeted to EGFR/HER2 exon 20 mutation, demonstrated favorable effect in EGFR/HER2 exon 20 insertion mutations. The therapeutic effect of poziotinib was more potent than afatinib in cell lines with HER2 exon 20 mutation (19). In a phase II study investigating the clinical activity of poziotinib in EGFR/HER2 exon 20 mutations, the ORR of HER2 subgroup was 50% (6/12) at 8 weeks (20). Compared to afatinib, poziotinib has smaller substituent and increased halogenation by the terminal benzene ring, to facilitate deeper binding of sterically hindered drug-binding pocket, which withholds structural changes from EGFR/HER2 exon 20 insertion mutations (19). Emerging in vitro study in line demonstrated that poziotinib was more efficacious against exon 19 L755P mutation and exon 20 insertion mutations than afatinib (21). Interestingly, resistant mechanism study identified the secondary C805S mutation at the covalent binding site of poziotinib to HER2 as a potential mechanism of acquired resistance (22). Pyrotinib, an irreversible pan-HER receptor tyrosine kinase inhibitor, also presented a superior anti-tumor effect than afatinib and ado-trastuzumab emtansine (T-DM1) in vitro, and has been proven effective in HER2-mutant NSCLC with an ORR of 53.3% and a mPFS of 6.4 months, without adverse events more than grade 3 (23). Of note, pyrotinib showed better tolerability and anti-cancer effect than afatinib at a relatively high dose (400 mg, p.o. once daily), where afatinib showed obvious dose-limiting toxicity. With an adjusted dose (400 mg, p.o. once daily), 6 of 10 (60%) patients harboring A775-G776insYVMA observed partial response. Even in rare mutations, L775P, G776>VC, G776C, pyrotinib exhibited clinical efficacy (23). Whereafter, Zhou and his colleagues confirmed the clinical activity of pyrotinib in a single-arm, phase II study, presenting an ORR of 31.67% (19/60), mPFS of 6.8 months (24). In addition, other TKIs exhibited little clinical activity. Neratinib monotherapy and combined therapy were investigated in a phase II study. The results showed that neratinib monotherapy had no clinical effect (0/17) on HER2 mutant patients, even combined with temsirolimus, the ORR was 18.6% (8/43) (25). The poor effect of neratinib was verified in a phase II basket trail targeting to HER2 mutations. In HER2 mutated lung cancer, the ORR was only 3.8% (1/26) (7). Similarly, no response (0/7) was observed in the HER2 exon 20 insertion mutation lung cancer treated with lapatinib (26). Collectively, these findings emphasized the fact that on top of different TKI agents, mutation subtypes also play a role as the biomarker to select patient for better clinical response to TKIs. Due to the encouraging anti-tumor activity from current available data, the therapeutic efficiency of pyrotinib and poziotinib in patients with NSCLC harboring HER2 exon 20 insertion mutations warrants to be validated in the future.
Trastuzumab monotherapy, demonstrated negative result in patients with immunohistochemistry (IHC) 3+ and IHC2+/dual color in situ hybridization (DISH)+, or in patients with A755_G776insYVMA and G776>VC on exon 20 and S310F mutation (27). Moreover, the effect of trastuzumab combined with gemcitabine–cisplatin was assessed in HER2 overexpressed NSCLC patients. Compared to 41% (21/51) ORR of control arm using trastuzumab monotherapy, the ORR of 36% (18/50) was surprisingly low. Importantly, 5 of 6 patients treated with the combination, with HER2 IHC 3+/fluorescence in situ hybridization (FISH)+, achieved partial response (28). In concert, this phenomenon was observed in another group of NSCLC patients with HER2 overexpression, trastuzumab plus carboplatin/paclitaxel performed greater in the HER2 IHC 3+ patients (29). Thus, IHC 1–2+ was not a reliable predicting biomarker in lung cancer. Of note, antibody-drug conjugate agent T-DM1, showed improved efficacy in HER2 mutations, presenting an ORR of 44.4% (8/18) (30). Referring the data of HER2 amplified NSCLC, 3 of 7 (43%) reached partial response (31). Collectively, T-DM1 might be a promising agent targeting HER2 mutated or amplified lung cancers.
There are several limitations of this study. In our analysis, the response with all specific genomic variants to afatinib monotherapy was not applicable because of incomplete data. Second, it is observed that most included studies were retrospective, concurrent oncogenic mutation like EGFR, ALK were not fully evaluated, which may influence the efficacy of afatinib. Third, study heterogeneity exists, referring as varied study designs, HER2 inspection methods and treatment lines. Most studies are retrospective with two are prospective. Afatinib was prescribed as backline therapy except the work by Al-Obeidi and colleagues. They treated the patients who refused standard chemotherapy with first-line afatinib (17). Peters and colleagues used afatinib on a compassionate basis because patients exhausted all other treatment (1).
The limited clinical activity of anti-HER2 agents to HER2 alterations may have diverse reasons. First, the approaches to measure HER2 overexpression, HER2 amplification and HER2 mutation varied, unified standards are required to clarify this point. Moreover, the pharmacology and pharmacokinetics aspects of TKIs, antibody and chemotherapy alone or in combinations should be further illuminated. Third, the primary and acquired resistance of anti-HER2 agents of three alterations need deeper understanding. Cumulatively, all those demonstrates that we may underestimate the complexity of HER2 alterations in NSCLC. We do not recommend the regular application of afatinib in the NSCLC with HER2 mutations unless further evidence concerning the optimal anti-HER2 approach in patients molecularly selected in a prudent way.
Table S1
| Adverse events | Peters (1), (N=28) | Dziadziuszko (13), (N=13) | Costa (15), (N=3) |
|---|---|---|---|
| Blood and lymphatic system disorders, n | 1 | 4 | |
| Serious adverse events, n (%) | |||
| Febrile neutropenia | 1 (7.7) | ||
| Not Serious adverse events, n (%) | |||
| Anemia | 2 (15.4) | ||
| Platelet count decreased | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Leukocytosis | 1 (3.6) | ||
| Cardiac and vascular disorders, n | 3 | ||
| Serious adverse events, n (%) | |||
| Pericardial effusion | 1 (7.7) | ||
| Not serious adverse events, n (%) | |||
| Ventricular arrhythmia | 1 (7.7) | ||
| Hypertension | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Gastrointestinal disorders, n | 12 | 25 | 3 |
| Serious adverse events, n (%) | |||
| Diarrhea | 1 (7.7) | ||
| Not serious adverse events, n (%) | |||
| Diarrhea | 11 (84.6) | 3 (100.0) | |
| Mucositis oral | 4 (30.8) | ||
| Abdominal pain | 3 (23.1) | ||
| Vomiting | 3 (23.1) | ||
| Constipation | 1 (7.7) | ||
| Dry mouth | 1 (7.7) | ||
| Nausea | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Diarrhea | 10 (35.7) | ||
| Vomiting/nausea | 2 (7.1) | ||
| Metabolism and nutrition disorders, n | 6 | ||
| Serious adverse events, n (%) | |||
| Dehydration | 1 (7.7) | ||
| Not serious adverse events, n (%) | |||
| Hyperkalemia | 1 (7.7) | ||
| Hypermagnesemia | 1 (7.7) | ||
| Hypoalbuminemia | 1 (7.7) | ||
| Hypomagnesemia | 1 (7.7) | ||
| Hyponatremia | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Musculoskeletal and connective tissue disorders, n | 5 | ||
| Serious adverse events, n (%) | |||
| Muscle weakness lower limb | 1 (7.7) | ||
| Not serious adverse events, n (%) | |||
| Arthralgia | 1 (7.7) | ||
| Back pain | 1 (7.7) | ||
| Bone pain | 1 (7.7) | ||
| Myalgia | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Renal and urinary disorders, n | 6 | ||
| Serious adverse events, n (%) | |||
| Acute kidney injury | 1 (7.7) | ||
| Not serious adverse events, n (%) | |||
| Cystitis noninfective | 1 (7.7) | ||
| Urinary incontinence | 1 (7.7) | ||
| Urinary track obstruction | 1 (7.7) | ||
| Bladder infection | 1 (7.7) | ||
| Urinary tract infection | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Respiratory, thoracic and mediastinal disorders, n | 3 | 10 | |
| Serious adverse events, n (%) | |||
| Dyspnea | 1 (7.7) | ||
| Epistaxis | 1 (7.7) | ||
| Pleural effusion | 1 (7.7) | ||
| Not serious adverse events, n (%) | |||
| Dyspnea | 3 (23.1) | ||
| Cough | 2 (15.4) | ||
| Epistaxis | 1 (7.7) | ||
| Pleural effusion | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Adult respiratory distress syndrome/dyspnea/lung infection/respiratory failure/respiratory insufficiency | 3 (10.7) | ||
| Eye disorders, n | 3 | ||
| Serious adverse events, n (%) | |||
| Not serious adverse events, n (%) | |||
| Dry eye | 2 (15.4) | ||
| Eye infection | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Skin and subcutaneous tissue disorders, n | 8 | 15 | 3 |
| Serious adverse events, n (%) | |||
| Not serious adverse events, n (%) | |||
| Erythema multiforme | 4 (30.8) | ||
| Rash acneiform | 4 (30.8) | 3 (100.0) | |
| Dry skin | 3 (23.1) | ||
| Other | 2 (15.4) | ||
| Alopecia | 1 (7.7) | ||
| Papulopustular rash | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Acne/dermatitis/dermatosis/pruritus/rash/skin toxicity | 8 (28.6) | ||
| Nervous system disorders, n | 2 | 5 | |
| Serious adverse events, n (%) | |||
| Not serious adverse events, n (%) | |||
| Headache | 2 (15.4) | ||
| Dysgeusia | 1 (7.7) | ||
| Peripheral sensory neuropathy | 1 (7.7) | ||
| Other (paraplegia from Th4) | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Paralysis | 1 (3.6) | ||
| Depressed consciousness | 1 (3.6) | ||
| Infections and infestations, n | 7 | 9 | |
| Serious adverse events, n (%) | |||
| Not serious adverse events, n (%) | |||
| Paronychia | 5 (38.5) | ||
| Sinusitis | 1 (7.7) | ||
| Tooth infection | 1 (7.7) | ||
| Nail infection | 1 (7.7) | ||
| Tonsillitis | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Septic shock | 1 (3.6) | ||
| Stomatitis/mucositis/mouth ulceration | 4 (14.3) | ||
| Paronychia | 2 (7.1) | ||
| General disorders, n | 10 | ||
| Serious adverse events, n (%) | |||
| Not serious adverse events, n (%) | |||
| Fatigue | 3 (23.1) | ||
| Flu like symptoms | 2 (15.4) | ||
| Non-cardiac chest | 2 (15.4) | ||
| Malaise | 1 (7.7) | ||
| Tumor pain | 1 (7.7) | ||
| Weight loss | 1 (7.7) | ||
| Adverse events of any grade, n (%) | |||
| Investigations, n | 4 | ||
| Serious adverse events, n (%) | |||
| Not serious adverse events, n (%) | |||
| GGT increased | 2 (15.4) | ||
| Aspartate aminotransferase increased | 1 (7.7) | ||
| Creatine increased | 1 (7.7) | ||
| Adverse events of any grade, n (%) |
GGT, gamma-glutamyltransferase.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peters S, Curioni-Fontecedro A, Nechushtan H, et al. Activity of afatinib in heavily pretreated patients with ERBB2 mutation-positive advanced NSCLC: findings from a Global Named Patient Use Program. J Thorac Oncol 2018;13:1897-905. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Rothschild SI. Targeted therapies in non-small cell lung cancer-beyond EGFR and ALK. Cancers (Basel) 2015;7:930-49. [Crossref] [PubMed]
- Oh IJ, Hur JY, Park CK, et al. Clinical activity of pan-HER inhibitors against HER2-mutant lung adenocarcinoma. Clin Lung Cancer 2018;19:e775-81. [Crossref] [PubMed]
- Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: a report from the Lung Cancer Mutation Consortium. Cancer 2017;123:4099-105. [Crossref] [PubMed]
- Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016;27:281-6. [Crossref] [PubMed]
- Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018;554:189-94. [Crossref] [PubMed]
- Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol 2015;26:1421-7. [Crossref] [PubMed]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [Crossref] [PubMed]
- Kosaka T, Tanizaki J, Paranal RM, et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res 2017;77:2712-21. [Crossref] [PubMed]
- Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Eng J, Hsu M, Chaft JE, et al. Outcomes of chemotherapies and HER2 directed therapies in advanced HER2-mutant lung cancers. Lung Cancer 2016;99:53-6. [Crossref] [PubMed]
- Dziadziuszko R, Smit EF, Dafni U, et al. Afatinib in NSCLC with HER2 mutations: results of the prospective, open-label phase II NICHE trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol 2019;14:1086-94. [Crossref] [PubMed]
- Lai WV, Lebas L, Barnes TA, et al. Afatinib in patients with metastatic or recurrent HER2-mutant lung cancers: a retrospective international multicentre study. Eur J Cancer 2019;109:28-35. [Crossref] [PubMed]
- Costa DB, Jorge SE, Moran JP, et al. Pulse afatinib for ERBB2 exon 20 insertion-mutated lung adenocarcinomas. J Thorac Oncol 2016;11:918-23. [Crossref] [PubMed]
- Ou SI, Schrock AB, Bocharov EV, et al. HER2 transmembrane domain (TMD) mutations (V659/G660) that stabilize homo- and heterodimerization are rare oncogenic drivers in lung adenocarcinoma that respond to afatinib. J Thorac Oncol 2017;12:446-57. [Crossref] [PubMed]
- Al-Obeidi E, Li T, Kelly K. Durable responses to afatinib as first-line therapy for HER2-mutated metastatic non-small-cell lung cancer. Clin Lung Cancer 2020;21:e15-20. [Crossref] [PubMed]
- Liu Z, Wu L, Cao J, et al. Clinical characterization of ERBB2 exon 20 insertions and heterogeneity of outcomes responding to afatinib in Chinese lung cancer patients. Onco Targets Ther 2018;11:7323-31. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Heymach J, Negrao M, Robichaux J, et al. A phase II trial of poziotinib in EGFR and HER2 exon 20 mutant non-small cell lung cancer (NSCLC): OA02. 06. J Thorac Oncol 2018;13:S323-4. [Crossref]
- Robichaux JP, Nilsson MB, Zhang F, et al. Identification of poziotinib alone or in combination with TDM1 as a pan-HER2 inhibitor. Cancer Res 2019;79:abstr347.
- Koga T, Kobayashi Y, Tomizawa K, et al. Activity of a novel HER2 inhibitor, poziotinib, for HER2 exon 20 mutations in lung cancer and mechanism of acquired resistance: an in vitro study. Lung Cancer 2018;126:72-9. [Crossref] [PubMed]
- Wang Y, Jiang T, Qin Z, et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol 2019;30:447-55. [Crossref] [PubMed]
- Gao G, Li X, Wang Q, et al. Single-arm, phase II study of pyrotinib in advanced non-small cell lung cancer (NSCLC) patients with HER2 exon 20 mutation. J Clin Oncol 2019;37:9089. [Crossref]
- Gandhi L, Besse B, Mazieres J, et al. MA04. 02 neratinib±temsirolimus in HER2-mutant lung cancers: an international, randomized phase II study. J Thorac Oncol 2017;12:S358-9. [Crossref]
- Lopez-Chavez A, Thomas A, Rajan A, et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J Clin Oncol 2015;33:1000-7. [Crossref] [PubMed]
- Kinoshita I, Goda T, Watanabe K, et al. 1491P A phase II study of trastuzumab monotherapy in pretreated patients with non-small cell lung cancers (NSCLCs) harboring HER2 alterations: HOT1303-B trial. Ann Oncol 2018;29:mdy292.112.
- Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 2004;15:19-27. [Crossref] [PubMed]
- Langer CJ, Stephenson P, Thor A, et al. Trastuzumab in the treatment of advanced non-small-cell lung cancer: is there a role? Focus on Eastern Cooperative Oncology Group study 2598. J Clin Oncol 2004;22:1180-7. [Crossref] [PubMed]
- Li BT, Shen R, Buonocore D, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol 2018;36:2532-7. [Crossref] [PubMed]
- Li BT, Makker V, Buonocore DJ, et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol 2018;36:2502. [Crossref]


