The safety of first and subsequent lines of PD-1/PD-L1 inhibitors monotherapy in non-small cell lung cancer patients: a meta-analysis
Introduction
Lung cancer was related to 18.4% of deaths, and also was the most lethal tumor which led to 11.6% new cases of cancer in 2018 (1). Among lung cancer, non-small cell lung cancer (NSCLC) accounts for 84% (2). Two decades ago, chemotherapy had shown a unique effect in the treatment of NSCLC. Platinum-based doublet therapy has been the standard therapy for patients with advanced-stage NSCLC and good performance status (3). After the researcher found the mechanisms of gene mutation and function of the immune system in tumorigenesis, the evolution in the treatment and other therapy of NSCLC went faster. Targeted therapy and immunotherapy have come out one after another. Immunotherapy facilitates the recognition of cancer as foreign by the host immune system, then stimulates the immune system, and relieves the inhibition that allows growth and spread of disease (4).
From the latest studies, some of the programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors have shown better efficacy than conventional chemotherapy in second-line therapy. Besides the better survival curve, PD-1/PD-L1 inhibitors are also safer than chemotherapy. As a result, nivolumab (5-11) pembrolizumab (12), atezolizumab (13-15) and durvalumab (16) have been approved by Food and Drug Administration (FDA) for the treatment of patients with metastatic NSCLC in the second-line. Following the pace of second-line researches, the studies of first-line treatment of PD-1/PD-L1 inhibitors are currently in full swing. Fortunately, some PD-1/PD-L1 inhibitors have also shown good efficacy in first-line treatment. As the only one PD-1/PD-L1 inhibitor approved in first-line monotherapy of NSCLC, pembrolizumab has shown better treatment benefit in the patients with ≥1% of tumor cells expressing PD-L1, especially the patients with ≥50% PD-L1 expression (17,18). Basing on the IMpower 150 trial (19), patients who have metastatic non-squamous NSCLC with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations received the first-line treatment of atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin. More researches about first-line therapy of PD-1/PD-L1 inhibitors are ongoing. Many clinical trials and meta-analysis have shown that PD-1/PD-L1 inhibitors have fewer side effects than chemotherapy, no matter in first-line or subsequent therapy (5,6,10-13,15,17,18,20-22). However, the safety and disease spectra of side effects in different therapy time might have heterogeneity.
To figure out if there were any heterogeneity for the safety between first-line and subsequent therapy of PD-1/PD-L1 inhibitors in patients with NSCLC, we assessed and compared the safety of PD-1/PD-L1 inhibitors in first or subsequent line therapy, providing the following information for different therapy lines including incidence of adverse events, toxicity spectra, system-specific disease spectra as well as the rates of discontinuation and death.
Methods
Literature search
We searched in databases comprehensively, including PubMed, Cochrane library, and Embase using the following terms in English: (PD-1 OR PD-L1 OR “programmed death receptor 1” OR “programmed death receptor ligand” OR “immune checkpoint inhibitor” OR nivolumab OR BMS936558 OR ONO-4538 OR pembrolizumab OR MK-3475 OR atezolizumab OR Tecentriq OR durvalumab OR Imfinzi OR avelumab) AND (“non-small cell lung cancer” OR NSCLC). The articles should be identified as randomized controlled trials with publication date before February 01, 2020.
Inclusion criteria and Exclusion criteria
The eligibility criteria used for selecting studies in this meta-analysis were as following: (I) prospective clinical trials that provide detailed information about treatment-related adverse events (trAEs) and immune-related adverse events (irAEs), including the total number of patients, cases, and full side effects; (II) all patients were diagnosed with stage III/IV NSCLC with Eastern Cooperative Oncology Group (ECOG) performance status score of 0/1; (III) the treatment must be the first-line or subsequent monotherapy of PD-1/PD-L1 inhibitors; (IV) studies need to be in English. We used not only the full text but also the appendix and the references of each article would be used as the information resources. Studies that failed to meet the criteria above were excluded from this analysis; at the same time, we have chosen those that offered the most up-to-date and detailed data information as the primary data sources for this research. The other different studies from the same trial would be kept as the sources of other information supplements.
Data extraction and quality assessment
Two authors (YL Yang and PL Pang) evaluated each article independently. If there were any different opinions, the difference would be discussed with the third author (HR Liang). The data collected includes first author’s name, publication, publish year, phase of trials, blind method, trial name, NCT number, drug, therapy line, dose of drugs, trAEs (any grade and grade 3 or higher) classified by version 4.0 of the Common Terminology Criteria for Adverse Events (CTCAE 4.0) (23) of the National Cancer Institute, irAEs (any grade and grade 3 or higher) classified by the guideline of managing toxicities associated with immune checkpoint inhibitors (24), the sample size included in safety study, the number of patients with trAEs/irAEs as well as the number of discontinuation and deaths. The incidence and its 95% confidence interval (95% CI) of toxicities is used as a primary indicator of safety.
Statistical analysis
The incidence and its 95% CI were assessed by meta-analysis. The disease spectra of trAEs and irAEs were assessed by ranking the incidence of toxicities. In this process, heterogeneity was evaluated by Q test and I2 statistic. I2 over 50% (I2>50%) and P less than 0.05 (P<0.05) were considered as being with high heterogeneity. The random-effects model was used to calculate the summary estimate with high heterogeneity. Otherwise the fixed-effects model would replace it. The chi-square test was used to evaluate the statistical difference between different incidence, and a p-value of less than 0.05 (P<0.05) was considered as statistical significance. All of the statistical analysis was achieved by Statistical Product and Service Solutions version 24.0 (SPSS 24.0) and R studio Version 1.1.442, using the meta-package.
Results
Study selection and characteristics
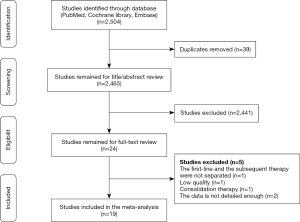
After the checking of duplicates, a total of 2,465 articles remained. By reading the titles and abstracts, 2,441 studies which were not meet the filter criteria were excluded from the research. Ultimately, after we carefully read the full text, we chose 19 articles (18 studies) (5-18,20,21,25-27). The summarizing process of study selection was showed in the flow chart (Figure 1), and the details of the included studies were listed in the following table (Table 1).
Table 1
| Author | Publication | Publish Year | Phase | Blind | Trial Name | Drug | Therapy line | Dose | Age (median) | No. of Patients† |
|---|---|---|---|---|---|---|---|---|---|---|
| Gettinger (25) | J Clin Oncol | 2016 | I | Open-label | CheckMate 012 | Nivolumab | First-line | 3 mg/kg Q2W | 67 | 52 |
| Carbone (20) | N Engl J Med | 2017 | III | Open-label | CheckMate 026 | Nivolumab | First-line | 3 mg/kg Q2W | 63 | 267 |
| Hellmann (26) | N Engl J Med | 2019 | III | Open-label | Checkmate 227 | Nivolumab | First-line | 240 mg Q2W | 64 | 391 |
| Borghaei (5) | N Engl J Med | 2015 | III | Open-label | Checkmate 057 | Nivolumab | Subsequence | 3 mg/kg Q2W | 61 | 287 |
| Brahmer (6) | N Engl J Med | 2015 | III | Open-label | Checkmate 017 | Nivolumab | Subsequence | 3 mg/kg Q2W | 62 | 131 |
| Gettinger (7) | J Clin Oncol | 2015 | I | Open-label | - | Nivolumab | Subsequence | 1, 3 or 10 mg/kg Q2W | 65 | 129 |
| Rizvi (8) | Lancet Oncol | 2015 | II | Open-label | CheckMate 063 | Nivolumab | Subsequence | 3 mg/kg Q2W | 65 | 117 |
| Hida (9) | Cancer Sci | 2017 | II | Open-label | - | Nivolumab | Subsequence | 3 mg/kg Q2W | 65 | 35 |
| Vokes (10) | Ann Oncol | 2018 | III | Open-label | Checkmate 017 and Checkmate 057 | Nivolumab | Subsequence | 3 mg/kg Q2W | 61 | 418 |
| Wu (11) | J Thorac Oncol | 2019 | III | Open-label | Checkmate 078 | Nivolumab | Subsequence | 3 mg/kg Q2W | 60 | 337 |
| Reck (17) | J Clin Oncol | 2019 | III | Open-label | Keynote-024 | Pembrolizumab | First-line | 200 mg Q3W | 64.5 | 154 |
| Mok (18) | Lancet | 2019 | III | Open-label | Keynote-042 | Pembrolizumab | First-line | 200 mg Q3W | 63 | 636 |
| Herbst (12) | Lancet | 2016 | II/III | Open-label | Keynote-010 | Pembrolizumab | Subsequence | 2 mg/kg Q2W, 10 mg/kg Q2W | 63 | 682 |
| Peters (14) | J Clin Oncol | 2017 | II | Open-label | BIRCH | Atezolizumab | First-line and Subsequence | 1,200 mg Q3W | 64 | 659 |
| Fehrenbacher (15) | Lancet | 2016 | II | Open-label | POPLAR | Atezolizumab | Subsequence | 1,200 mg Q3W | 62 | 142 |
| Rittmeyer (13) | Lancet | 2017 | III | Open-label | OAK | Atezolizumab | Subsequence | 1,200 mg Q3W | 63 | 609 |
| Gulley (27) | Lancet Oncol | 2017 | Ib | Open-label | JAVELIN Solid Tumor | Avelumab | Subsequence | 10 mg/kg Q2W | 65 | 184 |
| Barlesi (21) | Lancet | 2018 | III | Open-label | JAVELIN Lung 200 | Avelumab | Subsequence | 10 mg/kg Q2W | 64 | 393 |
| Garassino (16) | Lancet | 2018 | II | Open-label | ATLANTIC | Durvalumab | Subsequence | 10 mg/kg Q2W | 62 | 444 |
†, the number of patients calculated in the safety analysis.
trAEs
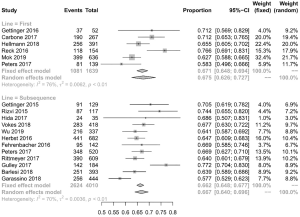
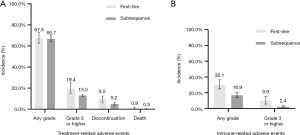
A total of 5,649 patients in 18 studies (6 of first-line therapy and 13 of subsequent therapy, Peters et al. (2017) (14) containing both first-line and subsequent therapy) were included in this analysis. The incidence of any-grade trAEs in different therapy line were similar (67.5% vs. 66.7%) (Figures 2A,S1, Table S1), with no statistically significant difference (P=0.710). As for high-grade trAEs, the incidence in first-line therapy was higher than the one of subsequent line (19.4% vs. 13.0%, P<0.001) (Figures 2A,S2, Table S1). In other words, first-line therapy was more accessible to lead to high-grade trAEs than subsequent therapy.
The common any-grade trAEs in first-line and subsequent therapy were similar, including fatigue, diarrhoea, nausea, and rash (Tables S2,S3). Besides, the incidence of aminotransferase increased was more obvious in first-line therapy. For high-grade trAEs, diarrhoea (1.1%), alanine aminotransferase increased (0.7%), pneumonitis (0.7%), rash (0.6%) and aspartate aminotransferase increased (0.5%) were the most frequent adverse events in first-line therapy (Table S4). Among the subsequent therapy, there were pneumonitis (0.8%), fatigue (0.8%), infusion-related reaction (0.2%), diarrhoea (0.2%) and γ-glutamyltransferase increased (0.1%) (Table S5).
irAEs
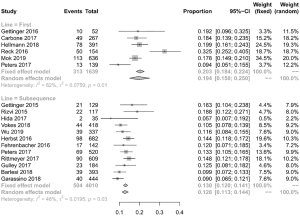
A total of 3,361 patients were evaluated across nine studies (two of first-line therapy, seven of subsequent therapy). Because only four studies in the subsequent therapy had mentioned the number of overall patients with irAEs, we calculated the overall incidence of irAEs in subsequent therapy from these four studies data. For the same reason, two of these four studies were incorporated in the analysis of high-grade irAEs. The morbidity of any-grade irAEs due to first-line therapy of PD-1/PD-L1 inhibitors in NSCLC patients was 30.1%. In comparison, the rate of subsequent therapy was 16.9% (Figures 2B,S3, Table S1) which is lower than the one of first-line therapy (P<0.001). In the analysis of high-grade irAEs, the incidence of first-line therapy was 9.9%, and the one of subsequent therapy was 2.4%. Alike the high-grade trAEs, the incidence of irAEs in first-line therapy was higher (P<0.001) (Figures 2B,S4, Table S1). Therefore, the relationship between first-line treatment and irAEs is closer than subsequent treatment, not only in any-grade irAEs but also in high-grade irAEs.
The most common any-grade irAEs of first-line therapy were hypothyroidism (11.7%), pneumonitis (8.2%), hyperthyroidism (6.3%), severe skin reactions (3.3%), and infusion reaction (3.0%) (Table S6). Meanwhile, any-grade irAEs of subsequent therapy were similar to the one of first-line therapy, including hypothyroidism (3.1%), pneumonitis (2.2%), rash (0.8%), hyperthyroidism (0.7%), and severe skin reactions (0.4%) (Table S7). As for the high-grade irAEs, the frequent adverse events were also similar such as pneumonitis and severe skin reactions. Besides these two irAEs, the common high-grade irAEs of first-line therapy also included hepatitis (1.1%), while colitis (0.1%) was ranked third in the subsequent therapy (Tables S8,S9).
Discontinue and death
Discontinuations due to trAEs were reported in 18 studies, happening in 350 of 5,183 patients. The discontinuation rate of first-line therapy was 9.5% (95% CI: 6.6–12.5%; I2=73% P<0.001) and the incidence for subsequent therapy was 5.2% (95% CI: 3.5–7.0%; I2=85% P<0.001). Death occurred in 42 of 5,205 patients, and the rate of deaths in first-line and subsequent therapy was 0.9% (95% CI: 0.4–1.5%; I2=38% P=0.15) and 0.5% (95% CI: 0.1–0.9%; I2=52% P=0.02), respectively (Figure 2A). The difference between the first-line and subsequent therapy of the rate of discontinuation is statistically significant (P<0.001). However, the statistical difference in the rate of death is not obvious.
System-specific disease spectra
According to CTCAE 4.0 (23) and the guideline of managing toxicities associated with immune checkpoint inhibitors (ICI) (24), we classified the adverse events to obtain the system-specific disease spectra of trAEs and irAEs in first-line or subsequent therapy of PD-1/PD-L1 inhibitors in NSCLC. For any-grade trAEs, the frequent system-specific adverse events that blame to PD-1/PD-L1 inhibitors for different therapy line were nearly the same. The typical treatment-related system-specific disease spectra at each grade in different therapy time were shown in Figure 3. Besides, the incidence of each system and the detailed systems of “others” were listed in the supplementary appendix (Tables S10-S13).
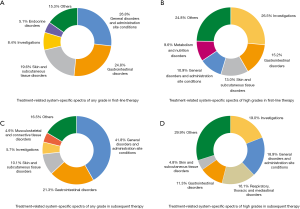
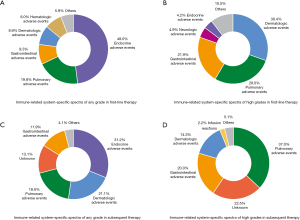
Notably, the spectra of system-specific irAEs were mostly different from those of trAEs. For any-grade irAEs, the most common system-specific irAEs were exactly alike between different therapy line. The common irAEs at each grade in different therapy time were shown in the figure below (Figure 4). More information was offered in the supplementary appendix (Tables S14-S17).
Discussion
Many trials have proved that PD-1/PD-L1 inhibitors have fewer side effects than chemotherapy (28). Recently, most PD-1/PD-L1 inhibitors have shown better efficacy and safety in subsequent therapy. Therefore, some clinical trials are working on the application of PD-1/PD-L1 inhibitors in first-line therapy (29). However, there has been no research on the comparison between the safety of PD-1/PD-L1 inhibitors in first-line and subsequent therapy. As more and more PD-1/PD-L1 inhibitors are approved in the first-line therapy, we believe that comparing the safety of first-line and subsequent therapy is conducive to the precision of treatment.
In this research, we found that first-line therapy was more likely to have adverse events than subsequent therapy, which may be related to two following reasons. Firstly, organisms had been changed after chemotherapy or other first-line therapy. Some studies have proved that chemotherapy may damage the immune system after the treatment (30). Meanwhile, PD-1 and PD-L1 targeting is an efficient way to maintain the function of effector T-cells (31), which is the primary mechanism of PD-1/PD-L1 inhibitors. Therefore, if the immune system had damaged by chemotherapy, the response of organisms to PD-1/PD-L1 inhibitors would probably be weaker. Secondly, the tumor of patients in different treatment lines had different degrees of malignancy or sensitivity to drugs. Entering the subsequent therapy means that the patients have a recurrence after remission of first-line therapy, or there is no relief after the first-line treatment, which shows that the tumor of these patients may be more malignant and less sensitive to the anti-tumor drugs. According to the mechanism mentioned above, this characteristic may also be one of the reasons for the difference between the incidence of adverse events in various therapy time.
Besides, some researches have shown that the irAEs of ICI are associated with its clinical outcome (32). Clinical trial data for each drug on different treatment lines showed that both overall survival (OS) and progress free survival (PFS) were higher in first-line therapy than subsequent therapy. Besides, the objective response rate (ORR) of first-line treatment is generally higher than the subsequent line. Nivolumab had an ORR of 23.0–26.0% in first-line treatment and 14.5–18.0% in second-line treatment (7-10,20,25,26). For pembrolizumab, the rate was 27.3–44.8% and 18% for first-line and second-line therapy, respectively (12,17,18).
Similarly, the rate of Atezolizumab was 22% for the first line and 14–19% for the subsequent line (13,14). It can be seen that a higher incidence of side effects in first-line treatment is not surprising. It also confirms our previous statement that the differences in the incidence of adverse events are related to the different responding degrees of organism and tumor to the drug. However, the incidence of any-grade trAEs in first-line therapy and subsequent therapy is similar, which is different from the results of high-grade trAEs, any-grade irAEs, and high-grade irAEs. We find that most of the common high-grade trAEs were also belong to irAEs, which is consistent with the result of previous studies (33). IrAEs are thought to result from either induction of autoimmunity or due to a proinflammatory state which means that the happening of irAEs is more related to the state of patients’ immune system (34). According to the content mentioned above, the insignificant difference in the incidence of any-grade trAEs may be attributable to the different mechanisms of common trAEs.
In terms of the spectra of adverse events, there were not many differences between first-line and subsequent therapy. Regarding trAEs, any-grade, and high-grade adverse events mainly related to the gastrointestinal tract, general conditions, skin and nutrition, among which the gastrointestinal adverse events were numerous and frequent. As for the irAEs, the endocrine system, respiratory system and skin were the most common systems involved. These consequences are consistent with the previous research (35). The result may affiliate with the mechanism of the emergence of adverse events due to PD-1/PD-L1 inhibitors.
According to recent articles, the following mechanisms were the reasons of the adverse events which was related to ICI, especially the irAEs: increasing T-cell activity against antigens that are present in tumors and healthy tissue, increasing levels of preexisting autoantibodies, increasing level of inflammatory cytokines, enhancing complement-mediated inflammation due to direct binding of a PD-1/PD-L1 antibody with PD-1/PD-L1 expressed on normal tissue. However, the reason for the system-specific phenomenon is still unclear (36).
Although there are many mechanisms, we can find that no matter in first-line or subsequent therapy, the common adverse events are similar, which means that the mechanisms leading to toxicities have not changed after first-line treatment with other therapies. Nevertheless, the incidence of pneumonitis ranked high in most of the analyses. Although the precise underlying mechanism remains unclear, some authors declared that alveolar macrophages might hyperactivate in patients receiving anti-PD-1 agents (37). Moreover, different from the subsequent therapy, the most common high-grade trAEs and irAEs in first-line therapy were both related to the liver according to the outcomes of specific adverse events. As the reason that the liver mainly metabolizes chemotherapy drugs, we consider that the main factor might be the damage led by the first-line therapy with other treatments as well, which has been mentioned above.
According to our results, there is some guiding significance for clinical medication of PD-1/PD-L1 inhibitors in the future. Firstly, we consider that patients with first-line therapy should be more alert to adverse effects. Secondly, clinicians should be alert to common side effects and should be adequately prepared and responded to promptly, such as the timely application of steroids. Thirdly, higher incidence of adverse events in first-line therapy prompts that for patients who are not in a good general condition, especially those with severe basal diseases in the gastrointestinal system, skin, respiratory system, thyroid gland and liver, the consideration of whether the benefit of first-line therapy is balanced with adverse reactions is necessary. Because there is a particular relationship between the efficacy of immunotherapy and the occurrence of side effects, side effects always exist in a typical proportion. At present, most of the coping methods are withdrawal. Therefore, for scientific research in the future, as most of the mechanisms of different adverse events are still unclear, the mechanisms should be studied in detail to manage the adverse events better.
However, this research still has some flaws. Firstly, the simples of first-line therapy and irAEs were not large enough, which might lead to deviations in results. Therefore, the meaning of the results on the two aspects are limited. We believe that, along with the progress of many trials about first-line therapy, there will be more authoritative and valuable results to provide more evidence for drug applications. Secondly, more and more researches and clinical applications tend to push PD-1/PD-L1 inhibitors into the direction of combination therapy. However, there are many types of combination therapy, and the mechanism of side effects caused by the combination is not very clear. Meanwhile, the number of trials of each kind of combination therapy is still limited. Because of the complexity and limited data of combination therapy, we currently chose to study the monotherapy of PD-1/PD-L1 inhibitors. In the future, to make the research more closer to clinical practice, it is valuable to assess the safety of combination therapy in different therapy lines.
Conclusions
In summary, the overall treatment-related safety of first-line therapy of PD-1/PD-L1 inhibitors in NSCLC is similar to the one of subsequent therapy. In contrast, the rate of having any-grade irAEs and high-grade adverse events in first-line therapy is higher. Meanwhile, there is no evident heterogeneity for disease spectra in different therapy lines.
Table S1
| Category (trAEs/irAEs and First/Subsequent line) | Any-grade Adverse Events | Grade 3 or Higher Adverse Events | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | Patients†/total‡ | Incidence | I2 | |||
| First-line therapy of trAEs | 1,081/1,639 | 0.675 [0.626; 0.727] | 76% | 313/1,639 | 0.194 [0.150; 0.250] | 82% | ||
| Subsequent therapy of trAEs | 2,624/4,010 | 0.667 [0.640; 0.696] | 70% | 504/4,010 | 0.130 [0.120; 0.141] | 48% | ||
| First-line therapy of irAEs | 229/790 | 0.301 [0.249; 0.364] | 57% | 71/790 | 0.099 [0.057; 0.151] | 70% | ||
| Subsequent therapy of irAEs | 289/1,703 | 0.169 [0.138; 0.207] | 70% | 15/628 | 0.024 [0.013; 0.037] | 0% | ||
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis. trAEs, treatment-related adverse events; irAEs, immune-related adverse events.
Table S2
| First line (Treatment-related adverse events) | Any-grade treatment-related adverse events | |||
|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | ||
| Fatigue | 187/1,500 | 0.151 [0.094; 0.218] | 90% | |
| Diarrhoea | 151/1,500 | 0.114 [0.068; 0.169] | 87% | |
| Rash | 141/1,500 | 0.100 [0.074; 0.128] | 60% | |
| Nausea | 108/1,500 | 0.081 [0.052; 0.116] | 77% | |
| Pruritus | 124/1,500 | 0.081 [0.067; 0.095] | 1% | |
| Decreased appetite | 113/1,500 | 0.068 [0.040; 0.102] | 78% | |
| Hypothyroidism | 89/1,500 | 0.030 [0.000; 0.102] | 97% | |
| Asthenia | 65/1,500 | 0.024 [0.005; 0.055] | 87% | |
| Aspartate aminotransferase increased | 65/1,500 | 0.022 [0.000; 0.074] | 95% | |
| Alanine aminotransferase increased | 65/1,500 | 0.021 [0.000; 0.072] | 95% | |
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis.
Table S3
| Subsequence (Treatment-related adverse events) | Any grade treatment-related adverse events | |||
|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | ||
| Fatigue | 510/3,490 | 0.151 [0.115; 0.191] | 89% | |
| Decreased appetite | 317/3,490 | 0.093 [0.055; 0.139] | 94% | |
| Nausea | 288/3,490 | 0.080 [0.049; 0.116] | 92% | |
| Diarrhoea | 185/3,490 | 0.052 [0.023; 0.090] | 95% | |
| Asthenia | 218/3,490 | 0.040 [0.017; 0.071] | 93% | |
| Rash | 173/3,490 | 0.033 [0.006; 0.078] | 97% | |
| Vomiting | 97/3,490 | 0.024 [0.009; 0.044] | 89% | |
| Pruritus | 132/3,490 | 0.019 [0.002; 0.048] | 95% | |
| Hypothyroidism | 142/3,490 | 0.018 [0.002; 0.045] | 95% | |
| Infusion-related reaction | 118/3,490 | 0.017 [0.000; 0.051] | 97% | |
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis.
Table S4
| First line (Treatment-related adverse events) | Grade 3 or higher treatment-related adverse events | |||
|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | ||
| Diarrhoea | 17/1,500 | 0.011 [0.003; 0.022] | 53% | |
| Alanine aminotransferase increased | 17/1,500 | 0.007 [0.000; 0.022] | 78% | |
| Pneumonitis | 23/1,500 | 0.007 [0.000; 0.024] | 81% | |
| Rash | 12/1,500 | 0.006 [0.002; 0.011] | 18% | |
| Aspartate aminotransferase increased | 12/1,500 | 0.005 [0.000; 0.018] | 74% | |
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis.
Table S5
| Subsequence (Treatment-related adverse events) | Grade 3 or higher treatment-related adverse event | |||
|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | ||
| Pneumonitis | 31/2,739 | 0.008 [0.003; 0.017] | 62% | |
| Fatigue | 29/2,739 | 0.008 [0.003; 0.015] | 57% | |
| Infusion-related reaction | 13/2,739 | 0.002 [0.000; 0.007] | 67% | |
| Diarrhoea | 13/2,739 | 0.002 [0.000; 0.005] | 46% | |
| γ-glutamyltransferase increased | 9/2,739 | 0.001 [0.000; 0.003] | 18% | |
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis.
Table S6
| First line (Immune-related adverse events) | Any Grade Immune-related Adverse Events | |||
|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | ||
| Hypothyroidism | 93/790 | 0.117 [0.095; 0.140] | 0% | |
| Pneumonitis | 65/790 | 0.082 [0.063; 0.101] | 0% | |
| Hyperthyroidism | 50/790 | 0.063 [0.046; 0.080] | 0% | |
| Severe skin reactions | 23/790 | 0.033 [0.007; 0.058] | 54% | |
| Infusion reaction | 18/790 | 0.030 [0.000; 0.064] | 73% | |
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis.
Table S7
| Subsequence (Immune-related adverse events) | Any grade immune-related adverse events | |||
|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | ||
| Hypothyroidism | 121/2571 | 0.031 [0.006; 0.073] | 95% | |
| Pneumonitis | 65/2571 | 0.022 [0.012; 0.036] | 73% | |
| Rash | 27/2571 | 0.008 [0.000; 0.025] | 91% | |
| Hyperthyroidism | 47/2571 | 0.007 [0.000; 0.024] | 91% | |
| Diarrhoea | 15/2571 | 0.004 [0.000; 0.015] | 84% | |
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis.
Table S8
| First line (Immune-related adverse events) | Grade 3 or higher immune-related adverse events | |||
|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | ||
| Pneumonitis | 26/790 | 0.034 [0.024; 0.049] | 0% | |
| Severe skin reactions | 19/790 | 0.031 [0.010; 0.090] | 84% | |
| Hepatitis | 8/790 | 0.011 [0.006; 0.022] | 0% | |
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis.
Table S9
| Subsequence (Immune-related adverse events) | Grade 3 or higher immune-related adverse events | |||
|---|---|---|---|---|
| Patients†/total‡ | Incidence | I2 | ||
| Pneumonitis | 26/2178 | 0.010 [0.006; 0.015] | 38% | |
| Severe skin reactions | 9/2178 | 0.001 [0.000; 0.006] | 69% | |
| Colitis | 5/2178 | 0.001 [0.000; 0.003] | 34% | |
†, the number of “patients” was the number of patients with the adverse events; ‡, the number of “Total” is the total number of patients who participated in the safety analysis in all articles included in the analysis.
Table S10
| Disorders | First line | |
|---|---|---|
| Any grade (%) | Proportion 95% CI (%) | |
| General disorders and administration site conditions | 30.40 | [18.48, 42.31] |
| Gastrointestinal disorders | 28.06 | [16.28, 39.84] |
| Skin and subcutaneous tissue disorders | 22.23 | [14.83, 29.63] |
| Investigations | 9.46 | [0.10, 18.82] |
| Endocrine disorders | 5.76 | [0.00, 14.22] |
| Nervous system disorders | 4.45 | [0.00, 4.59] |
| Blood and lymphatic system disorders | 3.94 | [0.46, 7.41] |
| Metabolism and nutrition disorders | 3.40 | [0.00, 9.27] |
| Respiratory, thoracic and mediastinal disorders | 2.51 | [0.00, 6.79] |
| Infections and infestations | 1.80 | [0.17, 3.42] |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 0.85 | [0.00, 2.39] |
| Cardiac disorders | 0.38 | [0.00, 1.45] |
| Eye disorders | 0.00 | [0.00, 0.00] |
| Hepatobiliary disorders | 0.00 | [0.00, 0.00] |
| Immune system disorders | 0.00 | [0.00, 0.00] |
| Injury, poisoning and procedural complications | 0.00 | [0.00, 0.00] |
| Musculoskeletal and connective tissue disorders | 0.00 | [0.00, 0.00] |
| Psychiatric disorders | 0.00 | [0.00, 0.00] |
| Renal and urinary disorders | 0.00 | [0.00, 0.00] |
| Vascular disorders | 0.00 | [0.00, 0.00] |
| unknown† | 0.00 | [0.00, 0.00] |
| Congenital, familial and genetic disorders | 0.00 | [0.00, 0.00] |
| Ear and labyrinth disorders | 0.00 | [0.00, 0.00] |
| Pregnancy, puerperium and perinatal conditions | 0.00 | [0.00, 0.00] |
| Reproductive system and breast disorders | 0.00 | [0.00, 0.00] |
| Social circumstances | 0.00 | [0.00, 0.00] |
| Surgical and medical procedures | 0.00 | [0.00, 0.00] |
†, disorders that can not be classified with Common Terminology Criteria for Adverse Events version 4.0.
Table S11
| Disorders | First line | |
|---|---|---|
| Grade 3–5 (%) | Proportion 95% CI (%) | |
| Investigations | 3.18 | [0.00, 7.54] |
| Gastrointestinal disorders | 1.83 | [0.29, 3.36] |
| Skin and subcutaneous tissue disorders | 1.56 | [0.00, 3.17] |
| General disorders and administration site conditions | 1.31 | [0.29, 2.33] |
| Metabolism and nutrition disorders | 1.15 | [0.00, 4.36] |
| Respiratory, thoracic and mediastinal disorders | 1.14 | [0.00, 2.82] |
| Blood and lymphatic system disorders | 0.67 | [0.84, 1.25] |
| Infections and infestations | 0.44 | [0.00, 1.48] |
| Cardiac disorders | 0.38 | [0.00, 1.45] |
| unknown† | 0.26 | [0.00, 0.98] |
| Endocrine disorders | 0.06 | [0.00, 0.24] |
| Musculoskeletal and connective tissue disorders | 0.03 | [0.00, 0.12] |
| Eye disorders | 0.00 | [0.00, 0.00] |
| Hepatobiliary disorders | 0.00 | [0.00, 0.00] |
| Immune system disorders | 0.00 | [0.00, 0.00] |
| Injury, poisoning and procedural complications | 0.00 | [0.00, 0.00] |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 0.00 | [0.00, 0.00] |
| Nervous system disorders | 0.00 | [0.00, 0.00] |
| Psychiatric disorders | 0.00 | [0.00, 0.00] |
| Renal and urinary disorders | 0.00 | [0.00, 0.00] |
| Vascular disorders | 0.00 | [0.00, 0.00] |
| Congenital, familial and genetic disorders | 0.00 | [0.00, 0.00] |
| Ear and labyrinth disorders | 0.00 | [0.00, 0.00] |
| Pregnancy, puerperium and perinatal conditions | 0.00 | [0.00, 0.00] |
| Reproductive system and breast disorders | 0.00 | [0.00, 0.00] |
| Social circumstances | 0.00 | [0.00, 0.00] |
| Surgical and medical procedures | 0.00 | [0.00, 0.00] |
†, disorders that can not be classified with Common Terminology Criteria for Adverse Events version 4.0.
Table S12
| Disorders | First line | |
|---|---|---|
| Any grade (%) | Proportion 95% CI (%) | |
| General disorders and administration site conditions | 39.04 | [28.38, 49.70] |
| Gastrointestinal disorders | 19.90 | [12.52, 27.28] |
| Skin and subcutaneous tissue disorders | 9.48 | [3.54, 15.42] |
| Investigations | 5.33 | [1.56, 9.11] |
| Musculoskeletal and connective tissue disorders | 4.32 | [1.85, 6.80] |
| Respiratory, thoracic and mediastinal disorders | 3.44 | [1.14, 5.73] |
| Blood and lymphatic system disorders | 3.20 | [1.71, 4.69] |
| Endocrine disorders | 2.66 | [0.58, 4.74] |
| Nervous system disorders | 2.15 | [0.51, 3.80] |
| Metabolism and nutrition disorders | 1.25 | [0.00, 2.66] |
| Immune system disorders | 1.17 | [0.00, 3.07] |
| Infections and infestations | 0.79 | [0.13, 1.45] |
| Vascular disorders | 0.30 | [0.01, 0.59] |
| unknown† | 0.25 | [0.00, 0.50] |
| Cardiac disorders | 0.09 | [0.00, 0.24] |
| Injury, poisoning and procedural complications | 0.04 | [0.00, 0.13] |
| Renal and urinary disorders | 0.04 | [0.00, 0.09] |
| Hepatobiliary disorders | 0.02 | [0.00, 0.06] |
| Psychiatric disorders | 0.02 | [0.00, 0.06] |
| Eye disorders | 0.00 | [0.00, 0.00] |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 0.00 | [0.00, 0.00] |
| Congenital, familial and genetic disorders | 0.00 | [0.00, 0.00] |
| Ear and labyrinth disorders | 0.00 | [0.00, 0.00] |
| Pregnancy, puerperium and perinatal conditions | 0.00 | [0.00, 0.00] |
| Reproductive system and breast disorders | 0.00 | [0.00, 0.00] |
| Social circumstances | 0.00 | [0.00, 0.00] |
| Surgical and medical procedures | 0.00 | [0.00, 0.00] |
†, disorders that can not be classified with Common Terminology Criteria for Adverse Events version 4.0.
Table S13
| Disorders | First line | |
|---|---|---|
| Grade 3–5 (%) | Proportion 95% CI (%) | |
| Investigations | 1.49 | [0.42, 2.56] |
| General disorders and administration site conditions | 1.48 | [0.63, 2.32] |
| Respiratory, thoracic and mediastinal disorders | 1.26 | [0.28, 2.23] |
| Gastrointestinal disorders | 0.89 | [0.30, 1.48] |
| Skin and subcutaneous tissue disorders | 0.38 | [0.00, 0.82] |
| Blood and lymphatic system disorders | 0.35 | [0.15, 0.55] |
| Vascular disorders | 0.29 | [0.00, 0.62] |
| Musculoskeletal and connective tissue disorders | 0.27 | [0.00, 0.56] |
| Metabolism and nutrition disorders | 0.25 | [0.00, 0.55] |
| Immune system disorders | 0.23 | [0.00, 0.56] |
| Infections and infestations | 0.23 | [0.02, 0.43] |
| Nervous system disorders | 0.19 | [0.00, 0.41] |
| unknown† | 0.18 | [0.00, 0.41] |
| Cardiac disorders | 0.14 | [0.00, 0.29] |
| Endocrine disorders | 0.14 | [0.00, 0.31] |
| Renal and urinary disorders | 0.04 | [0.00, 0.09] |
| Hepatobiliary disorders | 0.02 | [0.00, 0.06] |
| Psychiatric disorders | 0.02 | [0.00, 0.06] |
| Eye disorders | 0.00 | [0.00, 0.00] |
| Injury, poisoning and procedural complications | 0.00 | [0.00, 0.00] |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 0.00 | [0.00, 0.00] |
| Congenital, familial and genetic disorders | 0.00 | [0.00, 0.00] |
| Ear and labyrinth disorders | 0.00 | [0.00, 0.00] |
| Pregnancy, puerperium and perinatal conditions | 0.00 | [0.00, 0.00] |
| Reproductive system and breast disorders | 0.00 | [0.00, 0.00] |
| Social circumstances | 0.00 | [0.00, 0.00] |
| Surgical and medical procedures | 0.00 | [0.00, 0.00] |
†, disorders that can not be classified with Common Terminology Criteria for Adverse Events version 4.0.
Table S14
| Disorders | First line | |
|---|---|---|
| Any (%) | Proportion 95% CI (%) | |
| Endocrine adverse events | 20.30 | [14.15, 26.44] |
| Pulmonary adverse events | 8.38 | [0.94, 15.81] |
| Gastrointestinal adverse events | 3.93 | [0.00, 19.96] |
| Dermatologic adverse events | 3.78 | [0.00, 21.80] |
| Hematologic adverse events | 3.38 | [0.00, 26.40] |
| Rheumatologic/musculoskeletal adverse events | 0.97 | [0.00, 13.35] |
| Renal adverse events | 0.56 | [0.00, 1.69] |
| Neurologic adverse events | 0.56 | [0.00, 1.69] |
| Ophthalmologic adverse events | 0.32 | [0.00, 4.45] |
| Cardiovascular adverse events | 0.08 | [0.00, 1.08] |
| Unknown† | 0.00 | [0.00, 0.00] |
| Infusion reactions | 0.00 | [0.00, 0.00] |
†, disorders that can not be classified with the guideline of managing toxicities associated with immune checkpoint inhibitors.
Table S15
| Disorders | First line | |
|---|---|---|
| Grade 3–5 (%) | Proportion 95% CI (%) | |
| Dermatologic adverse events | 3.46 | [0.00, 25.48] |
| Pulmonary adverse events | 3.19 | [0.00, 10.66] |
| Gastrointestinal adverse events | 2.49 | [0.00, 12.13] |
| Neurologic adverse events | 0.56 | [0.00, 1.69] |
| Endocrine adverse events | 0.48 | [0.00, 2.61] |
| Renal adverse events | 0.40 | [0.00, 3.53] |
| Hematologic adverse events | 0.40 | [0.00, 3.53] |
| Ophthalmologic adverse events | 0.32 | [0.00, 4.45] |
| Cardiovascular adverse events | 0.08 | [0.00, 1.08] |
| Rheumatologic/musculoskeletal adverse events | 0.00 | [0.00, 0.00] |
| Unknown† | 0.00 | [0.00, 0.00] |
| Infusion reactions | 0.00 | [0.00, 0.00] |
†, disorders that can not be classified with the guideline of managing toxicities associated with immune checkpoint inhibitors.
Table S16
| Disorders | Subsequence | |
|---|---|---|
| Any grade (%) | Proportion 95% CI (%) | |
| Endocrine adverse events | 5.62 | [1.02, 10.23] |
| Dermatologic adverse events | 3.80 | [0.00, 9.29] |
| Pulmonary adverse events | 3.35 | [1.92, 4.79] |
| Unknown† | 2.36 | [0.00, 5.20] |
| Gastrointestinal adverse events | 2.14 | [0.00, 4.83] |
| Renal adverse events | 0.24 | [0.00, 0.84] |
| Rheumatologic/musculoskeletal adverse events | 0.18 | [0.00, 0.40] |
| Ophthalmologic adverse events | 0.16 | [0.00, 0.54] |
| Infusion reactions | 0.08 | [0.00, 0.27] |
| Neurologic adverse events | 0.07 | [0.00, 0.19] |
| Cardiovascular adverse events | 0.00 | [0.00, 0.00] |
| Hematologic adverse events | 0.00 | [0.00, 0.00] |
†, disorders that can not be classified with the guideline of managing toxicities associated with immune checkpoint inhibitors.
Table S17
| Disorders | Subsequence | |
|---|---|---|
| Grade 3–5 (%) | Proportion 95% CI (%) | |
| Pulmonary adverse events | 1.50 | [0.34, 2.65] |
| Unknown† | 0.91 | [0.00, 2.64] |
| Gastrointestinal adverse events | 0.81 | [0.00, 1.17] |
| Dermatologic adverse events | 0.58 | [0.00, 1.37] |
| Infusion reactions | 0.09 | [0.00, 0.32] |
| Neurologic adverse events | 0.09 | [0.00, 0.23] |
| Endocrine adverse events | 0.07 | [0.00, 0.26] |
| Renal adverse events | 0.00 | [0.00, 0.00] |
| Rheumatologic/musculoskeletal adverse events | 0.00 | [0.00, 0.00] |
| Ophthalmologic adverse events | 0.00 | [0.00, 0.00] |
| Cardiovascular adverse events | 0.00 | [0.00, 0.00] |
| Hematologic adverse events | 0.00 | [0.00, 0.00] |
†, disorders that can not be classified with the guideline of managing toxicities associated with immune checkpoint inhibitors.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.82). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Piccinni C, Calabria S, Ronconi G, et al. Facts and figures of clinical pathways in Italy: results from the PDTA Net project. Recenti progressi in medicina 2019;110:188-94. [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Shroff GS, de Groot PM, Papadimitrakopoulou VA, et al. Targeted Therapy and Immunotherapy in the Treatment of Non-Small Cell Lung Cancer. Radiologic clinics of North America 2018;56:485-95. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Hida T, Nishio M, Nogami N, et al. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non-small cell lung cancer. Cancer Sci 2017;108:1000-6. [Crossref] [PubMed]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. [Crossref] [PubMed]
- Wu YL, Lu S, Cheng Y, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol 2019;14:867-75. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Garassino MC, Cho B-C, Kim J-H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018;19:521-36. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018;19:1468-79. [Crossref] [PubMed]
- Nishijima TF, Shachar SS, Nyrop KA, et al. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist 2017;22:470-9. [Crossref] [PubMed]
- National Cancer Institute. Common terminology criteria for adverse events (CTCAE) Ver. 4.0. Bethesda: National Cancer Institute.
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18:599-610. [Crossref] [PubMed]
- Khan M, Lin J, Liao G, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97:e11936. [Crossref] [PubMed]
- Bylicki O, Barazzutti H, Paleiron N, et al. First-Line Treatment of Non-Small-Cell Lung Cancer (NSCLC) with Immune Checkpoint Inhibitors. BioDrugs 2019;33:159-71. [Crossref] [PubMed]
- Verma R, Foster RE, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res 2016;18:10. [Crossref] [PubMed]
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Frontiers in pharmacology 2017;8:561. [Crossref] [PubMed]
- Teraoka S, Fujimoto D, Morimoto T, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thorac Oncol 2017;12:1798-805. [Crossref] [PubMed]
- Wang Y, Zhou S, Yang F, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA oncology 2019;5:1008-19. [Crossref] [PubMed]
- Kaufman HL, Kirkwood JM, Hodi FS, et al. The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nature reviews Clinical oncology 2013;10:588-98. [Crossref] [PubMed]
- Wang PF, Chen Y, Song SY, et al. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Frontiers in pharmacology 2017;8:730. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Baraibar I, Melero I, Ponz-Sarvise M, et al. Safety and Tolerability of Immune Checkpoint Inhibitors (PD-1 and PD-L1) in Cancer. Drug Saf 2019;42:281-94. [Crossref] [PubMed]