
Increased serum level of interleukin-6 correlates with negative prognostic factors in extranodal NK/T-cell lymphoma
Introduction
Extranodal natural killer/T-cell lymphoma (ENKTL) is a highly aggressive malignant tumor with a poor prognosis (1). It is more common in Asia and Latin America than in Europe and North America (2), and accounts for 5% to 10% of malignant lymphomas in China (3). ENKTLs are histologically characterized by localized invasion, necrosis, and Epstein-Barr virus (EBV) infection of neoplastic cells. These tumors are confined to the nose and upper respiratory tract in 60% to 90% of patients (4), but may also occur outside the nasal cavity (e.g., in skin, testis, small intestine, and muscle) (2) or as a disseminated disease without any noticeable nasal involvement (5). Patients with extra-nasal ENKTL have a worse prognosis than those with nasal ENKTL (6).
Serum markers are related to tumor behavior. Some biomarkers - such as the plasma EBV DNA level at diagnosis (6), soluble interleukin 2 receptor (sIL2R) level (2,7), Chemokine (C-X-C motif) ligand 9 (CXCL9), CXCL10 (8), interleukin-15 (IL-15) (9), interleukin-2 (IL-2) (10), and interleukin 9 (IL-9) are associated with prognosis of ENKTL (11,12). In some hematologic malignancies, the presence of ferritin in the serum is the direct result of tumor activity (2). In addition, Ki67 index of greater than 50%, transformed tumor cells greater than 40%, an increased level of C-reactive protein (CRP) (13), anemia, and thrombocytopenia are used to predict the prognosis of ENKTL (6). However, serum level of IL-6 has been seldom interpreted in prognosis of ENKTL. IL-6 is a proinflammatory cytokine that promotes proliferation, survival, and activation of multiple lymphocyte lineages, the relation between IL-6 and ENKTL prognosis has not yet been clarified.
Methods
Patients
A retrospective cohort study was designed and patients with nasal ENKTL were systematically reviewed at the Department of Hematology of the First Affiliated Hospital of Zhejiang University between January 2010 and January 2018. A total of 236 newly diagnosed patients with ENKTL were enrolled in this study and 65 patients were selected. Inclusion criteria were (I) diagnosis of the World Health Organization classification of ENKTL; (II) CD3, CD56, and cytotoxic molecules were positive and CD20 was negative in situ hybridization; (III) no previous antitumor therapy; (IV) before treatment, serum samples were stored at the experimental center of the First Affiliated Hospital of Zhejiang University; and (V) complete follow-up results were available. Exclusion criteria were (I) diagnosis of other malignant tumors; (II) any medical coexisting problems that hindered the standard anti-tumor treatment; and (III) other subtypes of non-Hodgkin lymphoma (NHL), including acute myeloid/NK cell precursors of leukemia, naive NK cell lymphoma/precursor NK lymphocytic cell leukemia, aggressive NK cell leukemia, and peripheral T-cell lymphoma.
All the patients underwent physical examination, whole-body positron emission tomography/computed tomography CT (PET/CT) scan or magnetic resonance imaging (MRI) scan of head, neck, chest, abdomen and pelvis; routine blood examination; and blood biochemical examination. The study was approved by the Committee of the First Affiliated Hospital of Zhejiang University Medical School. All patients signed letters of informed consent before treatment. Clinical and laboratory characteristics were recorded including age, performance status, Ann Arbor stage, B symptoms, primary sites, sites of extranodal invasion, etc.
Treatment
Initial treatment of patients
The following treatment strategies were used: (I) early stage ENKTL patients received chemotherapy, followed by involved-field radiation therapy (IFRT). (II) Patients with advanced ENKTL received only chemotherapy. The non-L-asparaginase chemotherapy regimen consisted of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and EPOCH (etoposide, doxorubicin, cyclophosphamide, vincristine, and prednisone). Chemotherapy with L-asparaginase consisted of GELOX (gemcitabine, oxaliplatin L-asparaginase) or adjusted GELOX (14), SMILE (ifosfamide, methotrexate, L-asparaginase, and etoposide), CHOP-L (CHOP plus L-asparaginase) and DeVIC (dexamethasone, etoposide, ifosfamide, carboplatin). Patients received at least two cycles of initial chemotherapy and at most to eight cycles. The total dose of radiation therapy was 50–60 Gy in five fractions (1.8–2.0 Gy each) per week.
Cytokine detection
Serum levels of IL-2, IL-4, IL-6, IL-10, IL-17A, TNF-α, and interferon-γ (IFN-γ) were measured by enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems). All venous blood samples were obtained from 65 patients with ENKTL. Samples were collected, centrifuged at 4 °C, and frozen rapidly at −80 °C for further detection. The ELISA inspection was carried out according to instructions.
Evaluation of therapeutic efficiency
The treatment response was evaluated according to standardized criteria for NHL response (15). Patients were evaluated after two courses of chemotherapy.
Follow-up
After completion of treatment, the patients were evaluated by hematologists. Follow-up intervals were established according to routine criteria. The OS is from the time of the initial diagnosis until the time of death or until the last follow-up. progression-free survival (PFS) is the time of diagnosis until disease progression, recurrence, death from any cause, or until the last follow-up.
Statistics
Data statistics included nonparametric mean difference tests for the Mann-Whitney U-test. The concentration of cutoff was determined by the receiver operating characteristic (ROC) curve analysis. The correlation among LDH, IL-2, IL-4, IL-6, IL-10, IL-17A, TNF-α, IFN-γ, and complete remission was analyzed by Chi-square test. The effects of various factors on OS and PFS were calculated by using the Kaplan-Meier and log-rank test methods. The correlation between the factors was examined by using of the Spearman test. COX regression was utilized to analyze the prognostic significance of multivariate variables.
Results
Patient characteristics
The clinical features of 65 patients (48 males and 17 females, with a median age of 47 years old) were presented in Table 1. Among these patients, 46 (70.8%) had good Eastern Cooperative Oncology Group Performance Status (ECOG PS) (0–1), 44 (67.6%) had B symptoms (fever, unexplained temperature higher than 38 °C, night sweats, or weight loss within 6 months more than 10%), and 42 (64.6%) were Ann Arbor stage III/IV. The primary site was nasal cavity in 32 patients (49.2%) and the other 33 patients (50.8%) had extranasal sites. Ki67 index in 17 patients (26.2%) was more than 50%. Hemophagocytic syndrome was found in 12 patients (18.5%). At diagnosis, the median serum ferritin level was 422 µg/L (range, 65.5–1,829.00 µg/mL), the median CRP level was 10.3 mg/L (range, 0.5–154 mg/L), the median IL-2 level was 0.40 pg/mL (range, 0.01–27.74 pg/mL), the median IL-6 level was 10.06 pg/mL (range, 0.10–1,176.97 pg/mL), the median interleukin 10 (IL-10) level was 6.13 pg/mL (range, 0.1–5,447.32 pg/mL), and the median tumor necrosis factor-α (TNF-α) level was 0.95 pg/mL (range, 0.01–1,233.04 pg/mL).
Table 1
| Clinical features | Median [range] | Number (N=65) | Percentage (%) |
|---|---|---|---|
| Age | 47 [17–77] | ||
| ≤60 | 47 | 72.3 | |
| >60 | 18 | 27.7 | |
| Gender | |||
| Male | 48 | 73.8 | |
| Female | 17 | 26.2 | |
| Ann Arbor stage | |||
| I-II | 23 | 35.4 | |
| III-IV | 42 | 64.6 | |
| Primary site | |||
| Nasal | 32 | 49.2 | |
| Extranasal | 33 | 50.8 | |
| B symptom | |||
| No | 21 | 32.3 | |
| Yes | 44 | 67.6 | |
| ECOG PS | |||
| 0–1 | 46 | 70.8 | |
| ≥2 | 19 | 29.2 | |
| IPI score | |||
| 0–1 | 19 | 29.2 | |
| ≥2 | 46 | 70.8 | |
| Bone marrow involvement | |||
| No | 35 | 53.8 | |
| Yes | 30 | 46.2 | |
| Lymph node involvement | |||
| No | 30 | 46.2 | |
| Yes | 35 | 53.8 | |
| Ki67 | 0.7 [0.1–0.95] | ||
| >0.5 | 17 | 26.2 | |
| ≤0.5 | 48 | 73.8 | |
| EBV DNA (/L) | 5×10E3 [0–7.61×10E6] | ||
| Positive | 36 | 55.4 | |
| Negative | 15 | 29.4 | |
| Not detected | 14 | 21.5 | |
| WBC (10E9/L) | 3.8 [0.5–108.2] | ||
| <4 | 34 | 52.3 | |
| ≥4 | 31 | 447.7 | |
| HB (g/L) | 113 [55–161] | ||
| <110 | 31 | 47.7 | |
| ≥110 | 34 | 52.3 | |
| PLT (10E9/L) | 157 [5–519] | ||
| <100 | 19 | 29.2 | |
| ≥100 | 46 | 70.8 | |
| ALB (g/L) | 37 [10.3–53.1] | ||
| <35 | 25 | 38.5 | |
| ≥35 | 40 | 61.5 | |
| Cr (µmol/L) | 60 [4.7–179] | ||
| ≤85 | 61 | 93.8 | |
| >85 | 4 | 62 | |
| Hemophagocytosis | |||
| No | 53 | 81.5 | |
| Yes | 12 | 18.5 | |
| Ferritin (µg/L) | 422 [65.5–1,829.00] | ||
| CRP (mg/L) | 10.3 [0.5–154] | ||
| IL-2 (pg/mL) | 0.40 [0.01–27.74] | ||
| IL-4 (pg/mL) | 0.89 [0.01–20.48] | ||
| IL-6 (pg/mL) | 10.06 [0.10–1,176.97] | ||
| IL-10 (pg/mL) | 6.13 [0.1–5,447.32] | ||
| IL-17A (pg/mL) | 0.47 [0.01–63.27] | ||
| TNF-α (pg/mL) | 0.95 [0.01–1,233.04] | ||
| IFN-γ (pg/mL) | 2.09 [0.01–771.24] | ||
ENTKL, extranodal natural killer/T-cell lymphoma; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ.
Receiver operating characteristic curve analysis for the optimal cutoff point of lactate dehydrogenase, CRP, ferritin, and cytokines
To determine the best cutoff value, CRP, ferritin, LDH, and cytokines were analyzed by the ROC curve (Figure 1). For the LDH curve, the area under the curve (AUC) was 0.688 (0.561–0.816), the most differentiated cutoff value was 548 U/L, and sensitivity and specificity were 0.462 and 0.968, respectively. For the CRP curve, the AUC was 0.754 (0.637–0.871), the most differentiated cutoff value was 25.35 µg/L, and the sensitivity and specificity were 0.462 and 0.962, respectively. For the ferritin curve, the AUC was 0.707 (0.578–0.835), the most differentiated cutoff value was 323.25 mg/L, and the sensitivity and specificity were 0.795 and 0.577, respectively. For the IL-6 curve, the AUC was 0.833 (0.728–0.937), the most differentiated cutoff value was 15.92 pg/mL, and the sensitivity and specificity were 0.641 and 0.920, respectively. For the IL-10 curve, the AUC was 0.769 (0.651–0.888), the most differentiated cutoff value was 6.145 pg/mL, and the sensitivity and specificity were 0.692 and 0.800, respectively. For the TNF-α curve, the AUC was 0.728 (0.604–0.852), the most differentiated cutoff value was 0.275 mg/L, and the sensitivity and specificity were 0.846 and 0.520, respectively (Table 2).
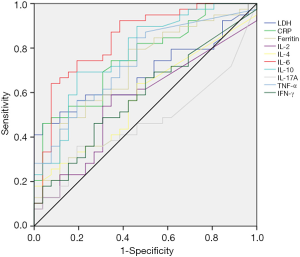
Table 2
| Variable | AUC | 95% CI | P value | Cutoff value | Sensibility | Specificity |
|---|---|---|---|---|---|---|
| LDH | 0.688 | 0.561–0.816 | 0.001 | 548 U/L | 0.462 | 0.968 |
| CRP | 0.754 | 0.637–0.871 | 0.001 | 25.35 µg/L | 0.462 | 0.962 |
| Ferritin | 0.707 | 0.578–0.835 | 0.005 | 323.25 mg/L | 0.795 | 0.577 |
| IL-2 | 0.552 | 0.408–0.696 | 0.487 | |||
| IL-4 | 0.570 | 0.429–0.711 | 0.346 | |||
| IL-6 | 0.833 | 0.728–0.937 | <0.001 | 15.920 pg/mL | 0.641 | 0.920 |
| IL-10 | 0.769 | 0.651–0.888 | <0.001 | 6.145 pg/mL | 0.692 | 0.800 |
| IL-17A | 0.481 | 0.339–0.622 | 0.794 | |||
| TNF-α | 0.728 | 0.604–0.852 | 0.002 | 0.275 pg/mL | 0.846 | 0.520 |
| IFN-γ | 0.588 | 0.446–0.729 | 0.239 |
LDH, lactate dehydrogenase; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ.
Efficacy and survival analysis
Chemotherapy was performed in 54 patients (83.1%), and the other 11 patients were treated with chemoradiotherapy. Among these patients, 45 (69.2%) received chemotherapy regimens containing L-asparaginase, and the remaining 20 (30.8%) received chemotherapy without L-asparaginase. Bone marrow transplantation was performed in 4 patients (6.2%), 35 patients (53.8%) achieved complete remission (CR), 6 patients (9.2%) achieved partial remission (PR), 6 patients (9.2%) were evaluated as having stable disease (SD), and 18 patients had progressive disease (PD).
CR rates for females and males were 76% and 46%, respectively (P=0.030). Patients with Ki-67 index more than 0.5 achieved a CR rate of 46%; Otherwise, CR rate was 76% (P=0.030). CR rate of patients with nasal primary sites was 78% and patients with extranasal sites was 30% (P<0.001). For LDH ≤548 U/L, the CR rate was 65%, and for LDH >548 U/L, CR rate was 26%, with significant difference (P=0.004). For Ferritin ≤323.25 and >325.25 µg/L, CR rates were 78% and 40%, respectively, with significant difference (P=0.003). For IL-6 ≤15.920 pg/mL and IL-6 >15.920 pg/mL, CR rates were 71% and 30%, respectively (P=0.001). For IL-10 ≤6.145 pg/mL and >6.145 pg/mL, CR rates were 73% and 34% (P=0.002) (Table 3).
Table 3
| Factor | Group | Number | CR rate (%) | P value |
|---|---|---|---|---|
| Gender | Female | 17 | 76 | 0.030 |
| Male | 48 | 46 | ||
| Age | ≤60 years | 47 | 60 | 0.139 |
| >60 years | 18 | 39 | ||
| Ki67 | ≤0.5 | 17 | 76 | 0.030 |
| >0.5 | 48 | 46 | ||
| LDH | ≤548 U/L | 46 | 65 | 0.004 |
| >548 U/L | 19 | 26 | ||
| ENV-DNA titer | ≤3.525×10E4/L | 32 | 66 | 0.006 |
| >3.525×10E4/L | 19 | 26 | ||
| CRP | ≤25.35 mg/L | 46 | 63 | 0.020 |
| >25.35 mg/L | 19 | 32 | ||
| Ferritin | ≤323.25 µg/L | 23 | 78 | 0.003 |
| >323.25 µg/L | 42 | 40 | ||
| IL-6 | ≤15.92 pg/mL | 38 | 71 | 0.001 |
| >15.92 pg/mL | 27 | 30 | ||
| IL-10 | ≤6.145 pg/mL | 33 | 73 | 0.002 |
| >6.145 pg/mL | 32 | 34 | ||
| TNF-α | ≤0.275 pg/mL | 19 | 53 | 0.901 |
| >0.275 pg/mL | 46 | 54 |
LDH, lactate dehydrogenase; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α; CR, complete remission.
Survival analysis
The median PFS was 282.23 days (9.41 months), and the median OS was 379.28 days (12.64 months) (Figures 2 and 3). Patients with Ann Arbor stage I-II and III-IV had significant differences in PFS and OS (P<0.001 and P=0.001, respectively). There were significant differences in PFS and OS in patients with nasal cavity ENKTL and extranasal ENKTL (P=0.001 and P=0.002, respectively). Patients with ECOG Performance Status (ECOG PS) score 0–1 and higher than 2 had significant differences in PFS and OS (P=0.001 and P<0.001, respectively). Patients treated with a chemotherapy regimen, with or without L-asparaginase, had significant differences in PFS and OS (P=0.021 and P=0.001, respectively). Patients with ferritin >323.25 µg/L and ferritin ≤323.25 µg/L, had significant differences in PFS and OS (P=0.001 and P<0.001, respectively). Patients with CRP ≤25.35 mg/L and CRP >25.35 mg/L had significant differences in PFS and OS (P=0.005 and P<0.001, respectively). Patients with IL-6 ≤15.920 pg/mL and IL-6 >15.920 pg/mL had significant differences in PFS and OS (P=0.002 and P<0.001, respectively). Patients with IL-10 ≤6.145 pg/mL and IL-10 >6.145 pg/mL had significant differences in PFS and OS (P=0.003 and P=0.001, respectively). There were no significant differences in PFS (P=0.086) and OS (P=0.806) in patients with TNF-α ≤0.275 pg/mL and TNF-α >0.275 pg/mL (Table 4 and Figures 2 and 3).
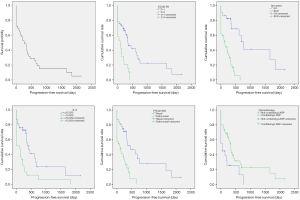
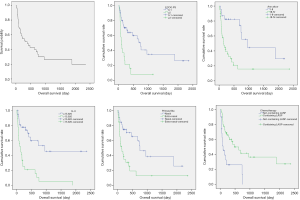
Table 4
| Factor | Progression-free survival | Overall survival | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||||
| P | HR | 95% CI | P | RR | 95% CI | P | HR | 95% CI | P | RR | 95% CI | ||||
| Age | |||||||||||||||
| ≤60 | |||||||||||||||
| >60 | 0.634 | 0.851 | 0.439–1.652 | 0.830 | 1.080 | 0.535–2.181 | |||||||||
| Gender | |||||||||||||||
| Female | |||||||||||||||
| Male | 0.924 | 1.032 | 0.546–1.947 | 0.728 | 1.129 | 0.569–2.241 | |||||||||
| Ann Arbor stage | |||||||||||||||
| I-II | |||||||||||||||
| III-IV | <0.001 | 5.664 | 2.409–113.316 | 0.001 | 6.011 | 2.102–17.191 | 0.001 | 3.515 | 1.637–7.546 | 0.015 | 3.600 | 1.278–10.141 | |||
| Primary site | |||||||||||||||
| Nasal | |||||||||||||||
| Extranasal | 0.001 | 3.035 | 1.571–5.864 | 0.778 | 11.127 | 0.49–2.591 | 0.002 | 2.870 | 1.479–5.567 | 0.865 | 1.086 | 0.420–2.809 | |||
| B symptom | |||||||||||||||
| No | |||||||||||||||
| Yes | 0.180 | 1.551 | 0.817–2.945 | 0.075 | 1.934 | 0.936–3.995 | |||||||||
| ECOG PS | |||||||||||||||
| 0–1 | |||||||||||||||
| ≥2 | 0.001 | 2.882 | 1.521–5.460 | 0.772 | 1.121 | 0.518–2.425 | <0.001 | 3.348 | 1.736–6.457 | 0.475 | 1.383 | 0.568–3.367 | |||
| IPI score | |||||||||||||||
| 0–1 | |||||||||||||||
| ≥2 | 0.025 | 2.262 | 1.107–4.625 | 0.125 | 1.795 | 0.850–3.791 | |||||||||
| Chemotherapy regime | |||||||||||||||
| No L-asparaginase | 0.021 | 2.096 | 1.116–3.937 | 0.061 | 1.969 | 0.970–3.984 | 0.001 | 3.077 | 1.577–5.988 | 0.009 | 2.717 | 1.252–5.780 | |||
| L-asparaginase | |||||||||||||||
| WBC (10E9/L) | |||||||||||||||
| <4 | |||||||||||||||
| ≥4 | 0.108 | 0.609 | 0.332–1.114 | 0.078 | 0.554 | 0.287–1.069 | |||||||||
| HB (g/L) | |||||||||||||||
| <110 | |||||||||||||||
| ≥110 | 0.869 | 0.952 | 0.533–1.703 | 0.629 | 0.856 | 0.457–1.606 | |||||||||
| PLT (10E9/L) | |||||||||||||||
| <100 | |||||||||||||||
| ≥100 | 0.054 | 0.533 | 0.281–1.011 | 0.023 | 0.459 | 0.235–0.898 | |||||||||
| LDH (U/L) | |||||||||||||||
| ≤548 | |||||||||||||||
| >548 | <0.001 | 4.371 | 2.157–8.856 | <0.001 | 4.669 | 2.292–9.510 | |||||||||
| Hemophagocytosis | |||||||||||||||
| No | |||||||||||||||
| Yes | 0.004 | 2.918 | 1.412–6.031 | 0.001 | 3.729 | 1.755–7.921 | |||||||||
| CRP (mg/L) | |||||||||||||||
| ≤25.35 | |||||||||||||||
| >25.35 | 0.005 | 2.371 | 1.290–4.356 | <0.001 | 3.564 | 1.883–6.748 | |||||||||
| Ferritin (µg/L) | |||||||||||||||
| ≤323.25 | |||||||||||||||
| >323.25 | 0.001 | 3.258 | 1.695–6.612 | <0.001 | 5.257 | 2.232–12.382 | |||||||||
| IL-6 (pg/mL) | |||||||||||||||
| ≤15.92 | |||||||||||||||
| >15.92 | 0.002 | 2.587 | 1.427–4.690 | 0.012 | 2.367 | 1.206–4.643 | <0.001 | 4.214 | 2.166–8.196 | 0.001 | 3.565 | 1.72–7.39 | |||
| IL-10 (pg/mL) | |||||||||||||||
| ≤6.145 | |||||||||||||||
| >6.145 | 0.003 | 2.531 | 1.373–4.667 | 0.001 | 4.419 | 2.168–9.005 | |||||||||
| TNF-α (pg/mL) | |||||||||||||||
| ≤0.275 | |||||||||||||||
| >0.275 | 0.086 | 2.035 | 0.903–4.586 | 0.806 | 2.155 | 0.896–5.181 | |||||||||
ENTKL, extranodal natural killer/T-cell lymphoma; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α.
Correlation analysis
A correlation analysis of LDH, ferritin, CRP, IL-6, and IL-10 was performed. Because the data are not a normal distribution, a Spearman correlation was carried out. There was a significant correlation between IL-6 and LDH, IL-6 and ferritin, IL-6 and CRP, IL-6 and IL-10. The correlation coefficient between IL-6 and LDH was 0.282, which was significant (P=0.023). The correlation coefficient between IL-6 and IL-10 was 0.544 (P<0.001). The correlation coefficient between IL-6 and ferritin was 0.363 (P=0.003). The correlation coefficient between IL-6 and CRP was 0.282, which was significant (P=0.023). Therefore, IL-6, Ann Arbor stage, primary sites, ECOG PS scores, and chemotherapy were included in the multivariate analysis.
Multivariate analysis
In the multivariate analysis of PFS, Ann Arbor stage [P=0.001, RR =6.011 (2.102–17.191)] and IL-6 [P=0.012, RR =2.367 (1.206–4.643)] were independent prognostic factors. In multifactor analysis of OS, Ann Arbor stage [P=0.015, RR =3.600 (1.278–10.141)], IL-6 [P=0.001, RR =3.565 (1.720–7.390)], and non-L-asparaginase chemotherapy [P=0.009, RR =2.717 (1.252–5.780)] were independent prognostic factors.
Discussion
This study demonstrated that an increased IL-6 level was significantly associated with poor survival outcomes with ENKTL patients. Our findings illustrated serum levels of cytokines IL-6 and IL-10, CRP, ferritin, LDH were related with prognosis of ENKTL, and IL-6 was correlated with the other factors. Ann Arbor stage I-II and III-IV, nasal cavity ENKTL and extra nasal ENKTL, ECOG Performance Status (ECOG PS) score 0–1 and higher than 2, chemotherapy with or without L-asparaginase had significant differences in PFS and OS of ENTKL patients. Therefore, we built a model with IL-6 level, Ann Arbor stage, primary site, ECOG PS and chemotherapy to predict prognosis for ENKTL.
Vose et al. reported an association among serum ferritin level, albumin, and CRP level. However, a multivariate model that used ferritin, albumin, and CRP levels did not predict patient outcome (16). Thus, the combination of inflammation factors could not predict prognosis. For this reason, we performed a correlation analysis of LDH, ferritin, CRP, IL-6, and IL-10, and found a significant correlation among IL-6 and the other serum markers.
Gene expression profiles of ENKTL tumor cells showed overexpression of chemokines and cytokines compared to normal NK cells (17). In patients with ENKTL, elevated serum levels of VEGF and IL-6 may be related to activation of the PI3K/Akt and JAK/STAT3 pathways in lymphoma cells, which in turn results in increased transcription of VEGF and IL-6 by phosphorylation of Akt and STAT3 (18). Whereas ENKTL is frequently associated with inflammation at diagnosis, the paracrine effect of tumor microenvironment can also be caused by the secretion of VEGF and IL-6 from inflammatory bone marrow cells and lymphocytes (19). Kim et al. reported that IL-6 correlated with OS in patients with ENKTL (20). Our study determined that IL-6 was an independent factor of PFS and OS in ENKTL patients.
In some hematologic malignancies, serum ferritin is directly related to tumor activity. Tisi et al. (21) reported that elevated level of ferritin was associated with increased hepcidin levels, which in turn indicated disease activity in patients with lymphoma. Patients with high ferritin level had a low CR rate and shorter PFS and OS (16), which was consistent with our findings (22).
Pretreatment serum IL-10 levels were believed to be associated with progression and prognosis of ENKTL. Moreover, IL-10 is an important immune-related cytokine that limits antitumor immunity, proliferation, and anti-apoptosis (14). Gravisaco et al. suggested that IL-10 was a key factor in the growth of mouse T-cell lymphoma cells (23). IL-10 also supports and enhances tumorigenicity (24) of T-cell lymphoma in the autocrine mode (25).
Treatment options for ENKTL included: radiotherapy, chemotherapy, chemotherapy followed by involved-field radiotherapy (IFRT), and hematopoietic stem cell transplantation (HSCT). ENKTL is sensitive to radiation therapy, and radiotherapy is the main treatment for stage I/II nasal NK/T cell lymphoma. Although a high remission rate can be achieved by radiotherapy alone, patients nonetheless experience a high rate of recurrence. Chemotherapy followed by IFRT is the standard treatment for ENKTL. L-asparaginase is regarded as the most important drug in treatments for advanced ENKTL. A meta-analysis showed that the use of L-asparaginase was associated with better overall response rate (ORR) and CR rates in both localized and systemic ENKTL (26). Pokrovsky et al. (27) reported a meta-analysis of L-asparaginase in newly diagnosed ENKTL, revealing that L-asparaginase-containing regimens were beneficial for early and advanced ENKTL, which corresponded to our findings.
The international prognostic index (IPI) considers patient age, disease stage, serum LDH level, extranodal lesion site numbers, and ECOG PS. In ENKTL, nearly 60% of patients belong to the low-IPI-risk group (0–1 points), but nevertheless show significant heterogeneity. In our study, a large number of our patients had advanced Ann Arbor stage and a high IPI score. Kim et al. (28) identified four risk factors (age, stage, non-nasal type and distant lymph-node involvement) that were independently prognostic of OS and PFS (29). Also, our study showed that non-nasal type ENKTL was a negative prognosis factor for PFS and OS.
In sum, our findings confirmed the relation between IL-6 and several clinical features of ENKTL. We concluded that serum IL-6, which can be easily measured in clinical practice, is an independent prognostic factor for this disease and offers a new insight into potential therapeutic strategies such as blockade of IL-6. Future prospective studies are warranted to confirm our findings.
Acknowledgments
The authors thank the patients and their families and all the investigators, including the physicians, nurses, and laboratory technicians in this study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Committee of the First Affiliated Hospital of Zhejiang University Medical School (No. IIT20200115A). All patients signed letters of informed consent before treatment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tse E, Kwong YL. NK/T-cell lymphomas. Best Pract Res Clin Haematol 2019;32:253-61. [Crossref] [PubMed]
- Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 2006;24:612-8. [Crossref] [PubMed]
- Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol 2012;138:429-34. [Crossref] [PubMed]
- Oshimi K, Kawa K, Nakamura S, et al. NK-cell neoplasms in Japan. Hematology 2005;10:237-45. [Crossref] [PubMed]
- Jo JC, Yoon DH, Kim S, et al. Clinical features and prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol 2012;89:103-10. [Crossref] [PubMed]
- Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 2009;113:3931-7. [Crossref] [PubMed]
- Wang L, Liao DZ, Zhang J, et al. Clinical significance of serum soluble interleukin-2 receptor-alpha in extranodal natural killer/T-cell lymphoma (ENKTL): a predictive biomarker for treatment efficacy and valuable prognostic factor. Med Oncol 2013;30:723. [Crossref] [PubMed]
- Moriai S, Takahara M, Ogino T, et al. Production of interferon-{gamma}-inducible protein-10 and its role as an autocrine invasion factor in nasal natural killer/T-cell lymphoma cells. Clin Cancer Res 2009;15:6771-9. [Crossref] [PubMed]
- Wang H, Zhu JY, Liu CC, et al. Increased serum levels of interleukin-15 correlate with negative prognostic factors in extranodal NK/T cell lymphoma. Med Oncol 2015;32:370. [Crossref] [PubMed]
- Chan KK, Shen L, Au WY, et al. Interleukin-2 induces NF-kappaB activation through BCL10 and affects its subcellular localization in natural killer lymphoma cells. J Pathol 2010;221:164-74. [Crossref] [PubMed]
- Nagato T, Kobayashi H, Kishibe K, et al. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients Clin Cancer Res 2005;11:8250-7. [Crossref] [PubMed]
- Zhang J, Wang WD, Geng QR, et al. Serum levels of interleukin-9 correlate with negative prognostic factors in extranodal NK/T-cell lymphoma. PloS one 2014;9:e94637. [Crossref] [PubMed]
- Hanakawa H, Orita Y, Sato Y, et al. Novel and simple prognostic index for nasal natural killer/T-cell lymphoma. Head Neck 2014;36:551-6. [Crossref] [PubMed]
- Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer 2013;119:348-55. [Crossref] [PubMed]
- Montes-Mojarro IA, Kim WY, Fend F, et al. Epstein - Barr virus positive T and NK-cell lymphoproliferations: Morphological features and differential diagnosis. Semin Diagn Pathol 2020;37:32-46. [Crossref] [PubMed]
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124-30. [Crossref] [PubMed]
- Lim SW, Ryu KJ, Lee H, et al. Serum IL18 is associated with hemophagocytosis and poor survival in extranodal natural killer/T-cell lymphoma. Leuk Lymphoma 2019;60:317-25. [Crossref] [PubMed]
- Burger R. Impact of interleukin-6 in hematological malignancies. Transfus Med Hemother 2013;40:336-43. [Crossref] [PubMed]
- Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms Nat Rev Cancer 2013;13:759-71. [Crossref] [PubMed]
- Kim SJ, Hong M, Do IG, et al. Serum survivin and vascular endothelial growth factor in extranodal NK/T-cell lymphoma, nasal type: implications for a potential new prognostic indicator. Haematologica 2015;100:e106-9. [Crossref] [PubMed]
- Tisi MC, Bozzoli V, Giachelia M, et al. Anemia in diffuse large B-cell non-Hodgkin lymphoma: the role of interleukin-6, hepcidin and erythropoietin Leuk Lymphoma 2014;55:270-5. [Crossref] [PubMed]
- Yamazaki E, Tomita N, Koyama S, et al. Serum ferritin level is prognostic of patient outcome in extranodal NK/T cell lymphoma, nasal type. Med Oncol 2014;31:149. [Crossref] [PubMed]
- Gravisaco MJ, Mongini C, Alvarez E, et al. IL-2, IL-10, IL-15 and TNF are key regulators of murine T-cell lymphoma growth. Int J Mol Med 2003;12:627-32. [PubMed]
- Hassuneh MR, Nagarkatti M, Nagarkatti PS. Role of interleukin-10 in the regulation of tumorigenicity of a T cell lymphoma Leuk Lymphoma 2013;54:827-34. [Crossref] [PubMed]
- Wang H, Wang L, Wuxiao Z, et al. Increased serum levels of interleukin-10 predict poor prognosis in extranodal natural killer/T-cell lymphoma patients receiving asparaginase-based chemotherapy. Onco Targets Ther 2015;8:2589-99. [PubMed]
- Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood 2018;131:2528-40. [Crossref] [PubMed]
- Pokrovsky VS, Vinnikov D. L-Asparaginase for newly diagnosed extra-nodal NK/T-cell lymphoma: systematic review and meta-analysis. Expert Rev Anticancer Ther 2017;17:759-68. [Crossref] [PubMed]
- Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol 2016;17:389-400. [Crossref] [PubMed]
- Allen PB, Lechowicz MJ. Management of NK/T-Cell Lymphoma, Nasal Type. J Oncol Pract 2019;15:513-20. [Crossref] [PubMed]


