Surgical and oncological outcomes after laparoscopic vs. open major hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the most frequent type of primary liver cancer, the sixth most commonly diagnosed cancer, and fourth dominating cause of cancer deaths worldwide according to 2018 global cancer statistics (1). Currently, the management of HCC depends mainly on multidisciplinary comprehensive treatment, of which surgical resection remains the prime therapeutic method for patients without distant metastasis (2).
Since the first laparoscopic liver resection (LLR) was reported in 1991 by Reich (3), the number of publications discussing LLR has increased, particularly over the past 10 years in Asia and Europe (4,5). With accumulated surgical experience and continuous improvements in laparoscopic devices and techniques, laparoscopic minor hepatectomy for tumors located in the anterolateral liver segments has become the standard practice (6). However, major resection for tumors located in the posterosuperior part of the liver (segments 1, 7, 8, and the superior part of segment 4) remain a laparoscopic challenge because of the limited visibility and difficulty controlling bleeding. Therefore, laparoscopic major hepatectomy (LMH) was deemed experimental in the Second International Consensus Conference Statement (7).
The precise definition of major hepatectomy is controversial, although most scholars define it as resection of greater or equal to 3 liver segments (8), namely, right hemihepatectomy, left hemihepatectomy, expanded hemihepatectomy, and central bisectionectomy. Furthermore, some experts recommended laparoscopic right posterior sectionectomy as part of LMH in the 2008 Louisville statement because this procedure is technically difficult (6). Recently, a number of clinical reports comparing LMH with open major hepatectomy (OMH) for HCC have been published, and the minimally invasive advantages of LMH have been recognized by some medical centers (9,10). However, most studies were retrospective or propensity-score-matched studies, and no high-quality randomized controlled trials have been conducted. Therefore, we carried out this meta-analysis by pooling the available nonrandomized comparative studies to compare the surgical and oncological outcomes of LMH vs. OMH for HCC in order to supply high-quality data for clinical practice.
Methods
This research was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11).
Data sources and search strategy
Two reviewers (Qian Lu and Nannan Zhang) implemented a systematic review of the literature independently. All studies published until April 2019 were identified by searching PubMed, Web of Science, Embase and the Cochrane Central Register of Controlled Trials, with the language limited to English. The keywords [(“laparoscopic” or “laparoscopy”) and (“major hepatectomy” or “major liver resection” or “right hepatectomy” or “right hemihepatectomy” or “right liver resection” or “left hepatectomy” or “left hemihepatectomy” or “left liver resection” or “right posterior sectionectomy” or “central hepatectomy” or “central bisectionectomy”) and (“hepatocellular carcinoma” or “primary liver cancer”)] were selected to identify all documents possibly associated with “LMH for HCC”. The reference lists of the identified studies were manually searched for extra studies.
Study selection and eligibility criteria
The inclusion criteria were: (I) studies with a clear definition of surgery as major hepatectomy, namely, right hepatectomy, left hepatectomy, central hepatectomy, or right posterior sectionectomy in accordance with the Brisbane 2000 Nomenclature 29 (12); (II) studies with a clear definition of the indications for HCC; and (III) studies reporting a clear description of the short- and long-term survival outcomes. The exclusion criteria were: (I) case reports, comments, letters, conference abstracts, review articles, non-English language studies, and nonhuman studies; (II) studies assessing outcomes of LMH alone or including less than 10 patients; (III) studies with similar patient data repeatedly published by the same author or institution; and (IV) studies including other minimally invasive approaches like laparoscopic-assisted, hand-assisted, hybrid techniques, single-site incision, robotic, or donor hepatectomies. Original data from the included studies were checked, analyzed, and combined.
Data extraction
All surgical and oncological outcomes of interest were collected, including operative time, intraoperative blood loss volume, blood transfusion rate, postoperative overall morbidity, major complications, postoperative mortality, hospital stay, margin distance, negative margin (R0) rate, 3- and 5-year DFS, and 3- and 5-year overall survival. Postoperative morbidity was graded by the Clavien classification model (13), and major complications were graded from III–V and were considered any condition requiring surgical, endoscopic, or radiological intervention, and any life-threatening complication (including central nervous system complications) requiring intermediate care or intensive care unit management and death of a patient. The following baseline characteristics were summarized: age, sex, sample size, operation type, study period, and conversion. Significant independent variables and external validity comparisons were summarized, namely, cirrhosis, Child-Pugh class, the retention rate of indocyanine green 15 min after administration (ICG-R15), microvascular invasion, and maximum tumor size.
Quality evaluation
The quality of every included study was evaluated using the Newcastle-Ottawa Quality Assessment Scale (14), which was developed for nonrandomized studies. Each study can be awarded up to 9 stars, consisting of 4 stars in patient selection, 2 stars in group comparability, and 3 stars in outcomes assessment. The total number of stars for all three components was assessed to evaluate the quality of the included studies. Studies with ≥6 total stars were considered high-quality.
Statistical analysis
Statistical analyses were implemented using Review Manager version 5.3.5 software (Cochrane Collaboration, Center, The Nordic Center, Copenhagen, Denmark). Risk differences (RD) with 95% confidence intervals (CIs) were used to assess the dichotomous variables, while weighted mean differences (WMD) with 95% CIs were used for continuous variables. If the study provided only medians and ranges, the means and standard deviations were estimated as depicted by Hozo et al. (15). The hazard ratio (HR) was utilized as a summary statistic for long-term survival outcomes. If the study data did not include HRs and 95% Cis, log HR and its standard error were estimated using Tierney et al.’s method (16). Cochran’s Q test was used to evaluate statistical heterogeneity, and severity of heterogeneity was assessed by I2 values, as recommended in the Cochrane Handbook (17) (0–40%: likely minimal; 30–60%: likely moderate; 50–90%: likely substantial; 75–100%: likely considerable). The random effects model was adopted if the I2 value was larger than 50% according to the DerSimonian-Laird method, otherwise, we used a fixed-effects model. Lastly, funnel plots were constructed to assess publication bias using informal visual inspection. Two-tailed P<0.05 was considered statistically significant.
Results
Search results and selection processes
According to our search formula, a total of 638 relevant published studies were initially identified. Among these studies, we excluded 621 articles based on their titles and abstracts because they didn’t satisfy our eligibility criteria. A comprehensive examination of the remaining 17 studies was conducted, and four were excluded (18-21) because they involved liver diseases other than HCC. Then remaining 13 studies were subjected to further quality assessment (22-34), and all were included in the final meta-analysis because their Newcastle-Ottawa scale scores were more than 6 points (Table 1). A flow chart of the selection processes is shown in Figure 1, which contains the reasons for excluding studies.
Table 1
| Author | Selection | Comparability | Outcome | Total stars | |||||
|---|---|---|---|---|---|---|---|---|---|
| (I) | (II) | (III) | (IV) | (V) | (VI) | (VII) | |||
| Chen et al. (22) | * | * | * | * | ** | * | – | – | 7 |
| Chen et al. (23) | * | * | * | * | ** | * | * | * | 9 |
| Cho et al. (24) | * | * | * | * | ** | * | * | – | 8 |
| Guro et al. (25) | * | * | * | * | ** | * | * | – | 8 |
| Kim et al. (26) | * | * | * | * | ** | * | * | – | 8 |
| Kim et al. (27) | * | * | * | * | ** | * | * | – | 8 |
| Komatsu et al. (28) | * | * | * | * | ** | * | * | – | 8 |
| Rhu et al. (29) | * | * | * | * | ** | * | * | – | 8 |
| Tarantino et al. (30) | * | * | * | * | ** | * | – | – | 7 |
| Xu et al. (31) | * | * | * | * | ** | * | * | * | 9 |
| Yoon et al. (32) | * | * | * | * | ** | * | * | * | 9 |
| Zhang et al. (33) | * | * | * | * | ** | * | * | – | 8 |
| Zhang et al. (34) | * | * | * | * | ** | * | * | – | 8 |
(I) Representativeness of the laparoscopic group; (II) selection of the open group; (III) exposure; (IV) outcome of interest not present at the start; (V) assessment of outcome; (VI) follow-up; (VII) adequacy of follow-up of the cohort; *, one score; **, two score.
Study characteristics and significant independent variables
Patients’ characteristics in the 13 included researches are summarized in Table 2. The studies involved a total of 1,225 cases from Italy, France, South Korea, and China, with 534 undergoing LMH (46.8%) and 691 undergoing OMH (53.2%). Studies were well-matched for age, sex, and surgical extension, and all were retrospective studies or propensity-score-matched studies. Of the 13 studies, three evaluated right hemi-hepatectomy, three evaluated right posterior sectionectomy, two evaluated left hemi-hepatectomy, and the remaining five studies evaluated both right and left hemi-hepatectomy or central bisectionectomy. The conversion rate to laparoscopy-assisted or open surgery ranged from 0–31.6% in eight studies, with 33 conversions totally (9.5%). In accordance with the principle of intention-to-treat, all converted patients were included in the LMH group.
Table 2
| Author | Country | Surgical extension | Study period | Study design | No. of patients | Age (y), (mean ± SD) | Sex (male/female) | Conversion, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMH | OMH | LMH | OMH | LMH | OMH | ||||||||
| Chen et al. (22) | China | Mixed | 2015–2016 | R | 126 | 133 | 50.8±10.6 | 50.3±11.9 | 93/33 | 108/25 | 3 (1.3) | ||
| Chen et al. (23) | China | RHH | 2007–2018 | PSM | 38 | 38 | 56.0±10.3 | 55.2±11.1 | 31/7 | 32/6 | 7 (18.4) | ||
| Cho et al. (24) | Korea | RPH | 2003–2012 | R | 24 | 19 | 53.9±12.6 | 60.0±8.9 | 17/7 | 16/3 | 3(12.5) | ||
| Guro et al. (25) | Korea | Mixed | 2004–2015 | R | 67 | 110 | 57.7±11.1 | 59.1±12.3 | 49/18 | 93/17 | NA | ||
| Kim et al. (26) | Korea | LHH | 2012–2016 | PSM | 37 | 37 | 57.6±11.3 | 54.8±11.8 | 30/7 | 31/6 | NA | ||
| Kim et al. (27) | Korea | Mixed | 2013–2015 | PSM | 18 | 36 | 55.7±13.2 | 54.6±12.8 | 13/5 | 22/14 | NA | ||
| Komatsu et al. (28) | France | Mixed | 2006–2014 | PSM | 38 | 38 | 61.5±12.2 | 61.7 ±16.1 | 34/4 | 33/5 | 12 (31.6) | ||
| Rhu et al. (29) | Korea | RPH | 2009–2016 | PSM | 53 | 97 | 58.0±8.8 | 58.2±9.4 | 43/10 | 81/16 | 5 (8.6) | ||
| Tarantino et al. (30) | Italy | RPH | 2000–2014 | R | 13 | 51 | 65.0±13.0 | 65.5±9.0 | 37/14 | 7/6 | 3 (23.1) | ||
| Xu et al. (31) | China | Mixed | 2015–2017 | PSM | 32 | 32 | 52.2±10.6 | 51.7±11.4 | 28/4 | 28/4 | NA | ||
| Yoon et al. (32) | Korea | RHH | 2007–2015 | PSM | 33 | 33 | 56.0±7.0 | 57.3 ±6.9 | 23/10 | 26/7 | NA | ||
| Zhang et al. (33) | China | RHH | 2010–2015 | R | 35 | 42 | 58.0±9.5 | 63.0 ±10.5 | 10/25 | 16/26 | 0 (0.0) | ||
| Zhang et al. (34) | China | LHH | 2012–2014 | R | 20 | 25 | 47.0±8.5 | 52.0 ±10.5 | 8/12 | 10/15 | 0 (0.0) | ||
LHH, left hemi-hepatectomy; RHH, right hemi-hepatectomy; RPS, right posterior sectionectomy; LMH, laparoscopic major hepatectomy; OMH, open major hepatectomy; PSM, propensity score matching study; R, retrospective study; SD, standard deviation; NA, not applicable.
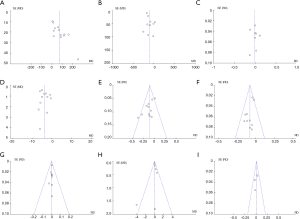
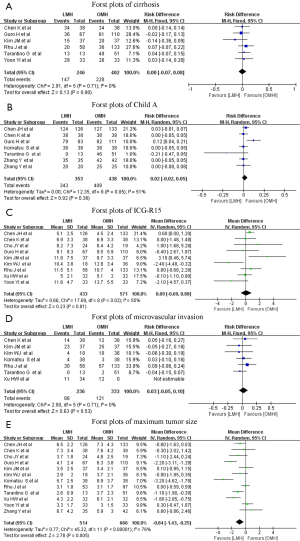
The significant independent variables of the included studies, namely, cirrhosis, Child–Pugh class A, ICG-R15, microvascular invasion, and maximum tumor size, are summarized in Table 3. The results of the pooled variables are summarized in Table 4, and additional details can be found in Figure S1. Results indicated no significant difference except for maximum tumor size. Only one study not report tumor size, and the pooled data showed that the maximum tumor size in patients undergoing LMH was smaller than in patients undergoing OMH (WMD: −0.84 cm; 95% CI: −1.43 to −0.25 cm; P=0.005), with substantial heterogeneity (I2=76%; P<0.00001).
Table 3
| Author | Cirrhosis, n (%) | Child A, n (%) | ICG-R15 (%) (mean ± SD) | Microvascular invasion, n (%) | Maximum tumor size (cm) (mean ± SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMH | OMH | LMH | OMH | LMH | OMH | LMH | OMH | LMH | OMH | |||||
| Chen et al. (22) | NA | NA | 124 (98.4) | 127 (95.5) | 5.1±2.5 | 4.5±2.4 | NA | NA | 6.5±2.2 | 7.3±4.3 | ||||
| Chen et al. (23) | 34 (89.5) | 34 (89.5) | 38 (100.0) | 38 (100.0) | 6.9±3.2 | 6.9±3.3 | 14 (36.8) | 12 (31.6) | 7.3±3.4 | 7.6±4.2 | ||||
| Cho et al. (24) | NA | NA | NA | NA | 8.2±7.3 | 6.4±4.2 | NA | NA | 3.7±1.8 | 4.8±2.5 | ||||
| Guro et al. (25) | 36 (54.5) | 61 (55.5) | 79 (95.2) | 92 (82.8) | 9.1±8.3 | 9.5±5.9 | NA | NA | 4.1±2.4 | 6.3±3.8 | ||||
| Kim et al. (26) | 15 (41.7) | 20 (54.1) | NA | NA | 11.8±7.5 | 8.7±3.3 | 23 (63.9) | 25 (67.6) | 3.5±2.5 | 3.4±2.1 | ||||
| Kim et al. (27) | NA | NA | NA | NA | 10.4±3.8 | 12.8±3.4 | 4 (22.2) | 10 (27.8) | 2.9±2.0 | 3.7±3.5 | ||||
| Komatsu et al. (28) | NA | NA | 38 (100.0) | 38 (100.0) | NA | NA | 4 (10.5) | 3 (7.9) | 5.7±2.5 | 8.9±3.7 | ||||
| Rhu et al. (29) | 20 (37.7) | 36 (37.1) | NA | NA | 11.5±5.1 | 10.7±4.0 | 30 (56.6) | 57 (58.8) | 3.1±1.8 | 3.1±1.7 | ||||
| Tarantino et al. (30) | 13 (100.0) | 49 (96.0) | 9 (69.2) | 46 (90.0) | NA | NA | 0 (0.0) | 2 (3.9) | 2.6±0.9 | 3.7±2.3 | ||||
| Xu et al. (31) | NA | NA | NA | NA | 5.0±2.1 | 5.1±2.0 | 11 (34.4) | 12 (37.5) | 4.3±2.2 | 6.1±2.1 | ||||
| Yoon et al. (32) | 29 (87.9) | 28 (80.7) | NA | NA | 11.6±4.7 | 13.7±5.5 | NA | NA | 3.3±1.7 | 3.0±1.5 | ||||
| Zhang et al. (33) | NA | NA | 35 (100.0) | 42 (100.0) | NA | NA | NA | NA | 6.7±4.2 | 5.9±3.0 | ||||
| Zhang et al. (34) | NA | NA | 20 (100.0) | 25 (100.0) | NA | NA | NA | NA | NA | NA | ||||
LMH, laparoscopic major hepatectomy; OMH, open major hepatectomy; Child A, Child–Pugh class A; ICG-R15, the retention rate of indocyanine green 15 min after administration; SD, standard deviation; NA, not applicable.
Table 4
| Variables | No. of studies | Effect model | No. of patients | Heterogeneity (P, I2) | Overall effect size | 95% CI of overall effect | P value | |
|---|---|---|---|---|---|---|---|---|
| LMH | OMH | |||||||
| Cirrhosis | 6 | Fixed | 246 | 402 | 0.71, 0% | RD =0.00 | (−0.07, 0.08) | 0.9 |
| Child A | 7 | Random | 353 | 438 | 0.05, 51% | RD =−0.002 | (−0.02, −0.05) | 0.36 |
| ICG-R15 | 9 | Random | 433 | 571 | 0.02, 55% | WMD =0.09 | (−0.69, 0.88) | 0.81 |
| Microvascular invasion | 7 | Fixed | 236 | 333 | 0.71, 0% | RD =0.03 | (−0.05, 0.10) | 0.53 |
| Maximum tumor size | 12 | Random | 514 | 666 | <0.00001, 76% | WMD =−0.84 | (−1.43, −0.25) | 0.005* |
| Operative time (min) | 13 | Random | 534 | 691 | <0.00001, 88% | WMD =72.14 | (43.07, 101.21) | <0.00001* |
| Blood loss (mL) | 11 | Random | 457 | 575 | <0.0001, 73% | WMD =−102.32 | (−150.99, −53.64) | <0.0001* |
| Blood transfusion | 8 | Random | 424 | 518 | 0.01, 61% | RD =−0.01 | (−0.06, 0.05) | 0.78 |
| Hospital stay (d) | 13 | Random | 481 | 594 | <0.00001, 76% | WMD =−3.77 | (−4.95, −2.60) | <0.00001* |
| Morbidity | 11 | Fixed | 479 | 624 | 0.09, 39% | RD =−0.01 | (−0.16, −0.06) | <0.00001* |
| Major complications | 10 | Fixed | 440 | 602 | 0.35, 10% | RD =−0.08 | (−0.11, −0.05) | <0.00001* |
| Mortality | 8 | Fixed | 375 | 438 | 0.99, 0% | RD =−0.01 | (−0.02, 0.01) | 0.57 |
| Margin distance (mm) | 6 | Fixed | 178 | 273 | 0.12, 43% | WMD =0.05 | (−0.1, 0.19) | 0.52 |
| R0 rate | 3 | Fixed | 172 | 162 | 0.40, 0% | RD =0.01 | (−0.03, 0.05) | 0.65 |
| 3-y disease-free survival | 8 | Fixed | 308 | 408 | 0.98, 0% | HR =0.99 | (0.72, 1.37) | 0.95 |
| 3-y overall survival | 8 | Fixed | 308 | 408 | 0.58, 0% | HR =1.25 | (0.70, 2.21) | 0.45 |
| 5-y disease-free survival | 3 | Fixed | 144 | 226 | 0.83, 0% | HR =0.94 | (0.64, 1.38) | 0.76 |
| 5-y overall survival | 3 | Fixed | 144 | 226 | 0.84, 0% | HR =0.94 | (0.45, 1.99) | 0.88 |
*, P<0.05 was considered statistically significant. CI, confidence interval; HR, hazard ratio; LMH, laparoscopic major hepatectomy; OMH, open major hepatectomy; Child A, Child-Pugh class A; ICG-R15, the retention rate of indocyanine green 15 min after administration; RD, risk difference; WMD, weighted mean difference.
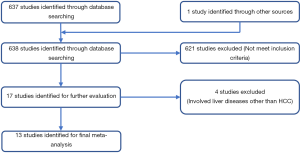
Meta-analysis for surgical outcomes
The pooled results of surgical outcomes are summarized in Table 4. Regarding intraoperative influences, operative time was recorded in all studies, and the pooled data showed a longer operative time in the LMH group (WMD: 72.14 minutes; 95% CI: 43.07–101.21 minutes; P<0.00001), with substantial heterogeneity between the studies (I2=88%; P<0.00001) (Figure 2A). In addition, blood loss data was available for eleven studies, and the pooled results indicated that LMH was related to less intraoperative blood loss vs. OMH (WMD: −102.32 mL; 95% CI: −150.99 to −53.64 mL; P<0.0001), with substantial heterogeneity between the studies (I2=73%; P<0.0001) (Figure 2B). Eight studies recorded the perioperative blood transfusion rate, and the pooled data indicated that the transfusion rate was not markedly different between LMH and OMH (RD: −0.01, 95% CI: −0.06 to 0.05, P=0.78), with substantial heterogeneity (I2=61%; P=0.01) (Figure 2C).
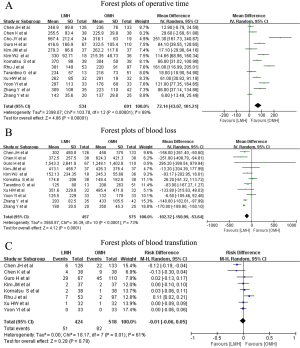
Regarding patients’ postoperative clinical course, hospital stay was pooled for all studies, and the pooled data indicated a significantly shorter hospital stay in the LMH group (WMD: −3.77 d; 95% CI: −4.95 to −2.60 d; P<0.0001), with substantial heterogeneity (I2=76%; P<0.00001) (Figure 3A). Eleven studies reported overall morbidity data; postoperative morbidity was 16.9% (90/534) after LMH and 30.1% (208/691) after OMH, and the pooled data showed a significantly lower postoperative overall morbidity in the LMH group (RD: −0.11; 95% CI: −0.16 to −0.06; P<0.0001) (Figure 3B), with minimal heterogeneity (I2=39%; P=0.09). Similar to overall morbidity, the pooled results for ten studies revealed that patients in the LMH group suffered fewer major complications (RD: −0.08; 95% CI: −0.11 to −0.05; P<0.00001) (Figure 3C), with minimal heterogeneity (I2=10%; P=0.35). Eight studies reported postoperative mortality, and the pooled results indicated no significant difference between LMH and OMH (RD: −0.01; 95% CI: −0.02 to 0.01; P=0.57), with minimal heterogeneity (I2=0%; P=0.99) (Figure 3D).
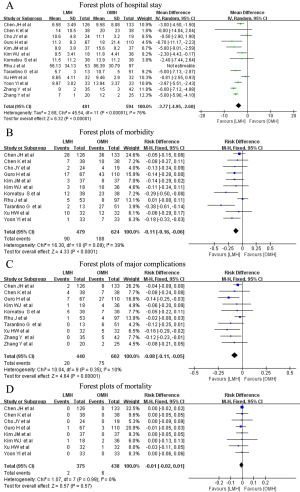
Meta-analysis for oncological outcomes
The pooled results of oncological outcomes are summarized in Table 4. Only five studies discussed the distance from the tumor margin, and margin distance was comparable between the two groups after pooling the results (WMD: 0.05 cm; 95% CI: −0.10 to 0.19 cm; P=0.52), with moderate heterogeneity (I2=43%; P=0.12) (Figure 4A). R0 resections was reported in three studies, and the pooled results indicated comparable outcomes between the two groups (RD: 0.01; 95% CI: −0.13 to 0.05; P=0.65), with minimal heterogeneity (I2=0%; P=0.40) (Figure 4B).

Regarding long-term outcomes, nine studies reported postoperative survival, and none found significant differences between the LMH and OMH groups in survival. Among the nine studies, 3- and 5-year survival rates were available in eight studies and three studies, respectively. The pooled results showed comparable 3-year DFS (HR: 0.99; 95% CI: 0.72–1.37; P=0.95) (Figure 5A), 3-year OS (HR =1.25; 95% CI: 0.70–2.21; P=0.45) (Figure 5B), 5-year DFS (HR: 0.94; 95% CI: 0.64–1.38; P=0.76) (Figure 5C), and 5-year OS (HR: 0.94; 95% CI: 0.45–1.99; P=0.88) (Figure 5D); heterogeneity was minimal (I2=0%; P>0.05).
Publication bias
Funnel plots for each perioperative outcome was drawn to evaluate symmetry (Figure 6). The drawn funnel plots were not asymmetrical, which suggested no or limited publication bias.
Discussion
Following the second International Laparoscopic Liver Surgery Expert Consensus Meeting (6,7), minor or non-anatomical LLR was the recommended standard practice, and some centers started to explore difficult major and anatomical LLR. Because of the complex biliary and vascular anatomy within the liver parenchyma and findings in diseases such as hepatitis and cirrhosis, it is especially difficult to perform major resection laparoscopically. However, with surgical experience and technical advances, some centers have achieved satisfactory surgical outcomes (35,36). Our meta-analysis revealed advantages with a laparoscopic approach regarding certain intraoperative and postoperative clinical measurements, but not regarding operative duration. In addition, our results highlighted that overall and disease-free survival (DFS) rates following LMH were comparable with those following OMH.
Regarding any surgical approach, patient safety is the most important issue, and our meta-analysis revealed no significant difference in postoperative mortality between two groups. Furthermore, our results showed that the laparoscopic approach achieved similar or better surgical outcomes compared with open surgery in major liver resection. Hemorrhage occurs easily during hepatectomy because hepatic vascular anatomy is complex, contains multiple blood sinuses, has abundant blood flow, and because the hepatic parenchyma is fragile. Therefore, effectively preventing intraoperative hemorrhage is an important factor in patient safety in laparoscopic surgery, and is the key to reducing rates of conversion to open surgery (37). Blood loss has a reported detrimental impact on postoperative death and liver dysfunction; therefore, the need for perioperative transfusion could indicate a worse poor prognosis (38). Our results showed less blood loss and similar transfusion rates with LMH for HCC, and the main reason may be that intra-abdominal pressure secondary to pneumoperitoneum plays an effective role in hemostasis. Additionally, the local magnified view helps surgeons precisely recognize the tiny blood vessels and bile ducts inside the liver parenchyma and more easily ligate or stop bleeding (39). Other significant factors for the lower blood loss with LMH are the ability to control blood flow, the choice of instruments and methods for liver transection, and the skilled manipulation secondary to extensive experience and the steep learning curve (40,41). Despite these factors, significant heterogeneity in blood loss and transfusion rates was documented in our meta-analysis. Therefore, we cannot exclude the fact that the advantages of a laparoscopic approach may be underestimated regarding blood loss and transfusion rates, or the reverse.
Regarding operation duration for LMH, which differs from similar or even reduced time with laparoscopic non-anatomical or minor resection (42,43), we found longer operative times with LMH for HCC, in our meta-analysis. The main reason may be the longer times required for the following with LMH: porta hepatis dissection, duct and vessel isolation, parenchymal resection, and bleeding control (10,44). In our pooled results, LMH operation duration was approximately 72 minutes longer than that for open procedures; a difference that is believed to have a little impact on patients’ postoperative outcomes. However, we found significant heterogeneity between LMH and OMH; therefore, a standardized surgical procedure throughout the learning curve is required. Furthermore, the average conversion rate was 9.5% (range, 0–31.6%) in our included studies, which was clearly higher than that reported in laparoscopic minor liver resection and reflects the higher technical demands during LMH. Generally, cirrhosis and intraoperative bleeding were the principal causes of conversion in most LMH processes (45). Moreover, preoperative treatments that warrant particular technical modifications, such as transarterial chemoembolization or portal vein embolization, are continued significant risk factors for conversion (28). Experience from large research centers showed that comprehensive patient assessment using preoperative imaging studies and intraoperative ultrasonography, determining tumor characteristics, and evaluating patients’ underlying liver condition could help lower the rate of conversion (22). Other studies have emphasized that a stepwise approach is appropriate to avoid conversions when performing laparoscopic liver surgery and recommend early conversion when encountering technical difficulties (37,46). Without doubt, the technical difficulty of LMH is a challenge for hepatobiliary surgeons; requiring sufficient experience and patience.
The ultimate goal of laparoscopic surgery highlights minimal invasiveness characterized by faster recovery and less complications. As expected, our pooled data showed better short-term postoperative outcomes. Compared with OMH, LMH for HCC was related to lower postoperative overall morbidity as well as fewer major complications. As with other laparoscopic procedures, LMH involves small abdominal incisions that minimize damage to the collateral circulation of the abdominal wall and lymphatic flow to the diaphragm; thus, reducing the incidence of refractory ascites (47). Moreover, the clearer surgical view provided by laparoscopy allows surgeons to ligate or stop bleeding more accurately; thus, reducing the incidence of postoperative hemorrhage and bile leakage. In addition to liver-specific complications, major liver resection has a damaging effect on respiratory function. However, we found in several included reports that pulmonary complications rate in patients undergoing LMH was lower than that in patients undergoing OMH (22,31). In addition, the minimally invasive advantage of LMH is reflected in patients’ lower postoperative pain, which facilitates early return to normal activities and gastrointestinal function recovery (48). We also saw a shorter hospitalization duration after LMH, in our meta-analysis, although significant heterogeneity was present between studies, possibly because of differences in medical insurance policies or postoperative management in different countries and institutions.
Surgical margins are an important factor affecting the prognosis of HCC patients. Because it is impossible to directly touch the liver or tumors located within the liver parenchyma, surgeons have difficulty visually judging the tumor boundaries with a laparoscopic approach, which stresses the need for preoperative and intraoperative imaging assessments such as three-dimensional reconstruction and intraoperative ultrasound (49,50). However, the ideal surgical margin is controversial. The Japanese guidelines recommend no tumor exposure (51), but some studies found that a margin >1 cm can reduce the recurrence rate of HCC (52). In our meta-analysis, the surgical margin and R0 rate for LMH were comparable to those for OMH, and the average surgical margin for all included reports was >1 cm, and was even >8 cm in the report by Kim (27). Consequently, our results showed no effect on the surgical margin using a laparoscopic approach, and the best surgical margin remains an important topic for discussion.
The long-term survival rate is conclusive for assessing LMH for HCC as a radical tumor surgery. As with previous studies of minor LLR (53,54), our study confirmed similar results for LMH, as we found no differences between LMH and OMH regarding long-term survival. Therefore, we believe that using laparoscopic techniques is not a direct factor, but that intraoperative hemorrhage, tumor exposure, and dissemination caused by a laparoscopic approach are the key factors affecting tumor recurrence and survival in HCC patients. Skilled laparoscopic techniques and extensive hepatectomy experience are prerequisites for LMH.
This meta-analysis included 13 studies published in the last 10 years and was based on a previous study (10); however, certain limitations warrant consideration. First, in our study, the tumor diameters in the LMH group were smaller than those in the OMH group, suggesting that some researchers were more conservative in selecting patients during the LMH learning phase, which may have underestimated the difficulty of LMH. Additionally, selection bias and unmeasured confounding factors were present in this meta-analysis because of deficiencies in retrospective studies, which may have impacts on outcomes. Second, the clinical heterogeneity caused by the perioperative management in different institutions may have influenced perioperative outcomes, especially reflected in operative time and hospital stay. Clinical heterogeneity caused by surgeons’ skill level also had reasonably influenced for perioperative outcomes, reflected in the intraoperative blood loss volume. Third, this meta-analysis compared a limited number of postoperative indicators. However, other perioperative outcomes that would have been useful in this meta-analysis varied in the majority of included studies, namely, the duration of patients’ intensive care unit stay, and readmission rates. Fourth, several studies showed that surgeons must perform LMH in 45–60 patients to overcome the learning curve (55,56). Unluckily, the number of cases in majority of the included studies are within the surgeons’ learning curve, and therefore our outcomes represent outcomes following LMH only during the learning period. In addition, although LMH has several advantages, the current research only included high-volume laparoscopic liver surgery centers; low-volume liver surgery centers may require a steeper learning curve.
Conclusions
Our meta-analysis results suggested that LMH can be carried out as safely as OMH in select patients and was associated with improved short-term outcomes, namely, less blood loss, lower postoperative morbidity, and shorter hospital stay, without affecting long-term survival. However, our results require confirmation in studies with high-quality designs and in prospective randomized controlled trials.
Acknowledgments
We thank Jane Charbonneau, DVM, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Gadsden MM, Kaplan DE. Multidisciplinary Approach to HCC Management: How Can This Be Done? Dig Dis Sci 2019;64:968-75. [Crossref] [PubMed]
- Reich H, Mcglynn F, Decaprio J, et al. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956-8. [PubMed]
- Kaneko H, Otsuka Y, Kubota Y, et al. Evolution and revolution of laparoscopic liver resection in Japan. Ann Gastroenterol Surg 2017;1:33-43. [Crossref] [PubMed]
- Yan Y, Cai X, Geller DA. Laparoscopic Liver Resection: A Review of Current Status. J Laparoendosc Adv Surg Tech A 2017;27:481-6. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854-62; discussion 862-4. [Crossref] [PubMed]
- Kasai M, Cipriani F, Gayet B, et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery 2018;163:985-95. [Crossref] [PubMed]
- Chen K, Pan Y, Hu GY, et al. Laparoscopic Versus Open Major Hepatectomy for Hepatocellular Carcinoma: A Meta-Analysis. Surg Laparosc Endosc Percutan Tech 2018;28:267-74. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg 2013;257:377-82. [Crossref] [PubMed]
- Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009;250:798-807. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Higgins JPT, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration;2011. Available online: http://handbook.cochrane.org, accessed March 7, 2018.
- Cho HD, Kim KH, Hwang S, et al. Comparison of pure laparoscopic versus open left hemihepatectomy by multivariate analysis: a retrospective cohort study. Surg Endosc 2018;32:643-50. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Laparoscopic major hepatectomies: current trends and indications. A comparison with the open technique. Updates Surg 2015;67:157-67. [Crossref] [PubMed]
- Medbery RL, Chadid TS, Sweeney JF, et al. Laparoscopic vs open right hepatectomy: a value-based analysis. J Am Coll Surg 2014;218:929-39. [Crossref] [PubMed]
- Kang SH, Kim KH, Shin MH, et al. Surgical outcomes following laparoscopic major hepatectomy for various liver diseases. Medicine (Baltimore) 2016;95:e5182. [Crossref] [PubMed]
- Chen J, Li H, Liu F, et al. Surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore) 2017;96:e6460. [Crossref] [PubMed]
- Chen K, Pan Y, Wang YF, et al. Laparoscopic Right Hepatectomy for Hepatocellular Carcinoma: A Propensity Score Matching Analysis of Outcomes Compared with Conventional Open Surgery. J Laparoendosc Adv Surg Tech A 2019;29:503-12. [Crossref] [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Outcomes of laparoscopic right posterior sectionectomy in patients with hepatocellular carcinoma in the era of laparoscopic surgery. Surgery 2015;158:135-41. [Crossref] [PubMed]
- Guro H, Cho JY, Han HS, et al. Outcomes of major laparoscopic liver resection for hepatocellular carcinoma. Surg Oncol 2018;27:31-5. [Crossref] [PubMed]
- Kim JM, Kwon CHD, Yoo H, et al. Which approach is preferred in left hepatocellular carcinoma? Laparoscopic versus open hepatectomy using propensity score matching. BMC Cancer 2018;18:668. [Crossref] [PubMed]
- Kim WJ, Kim KH, Kim SH, et al. Laparoscopic Versus Open Liver Resection for Centrally Located Hepatocellular Carcinoma in Patients With Cirrhosis: A Propensity Score-matching Analysis. Surg Laparosc Endosc Percutan Tech 2018;28:394-400. [Crossref] [PubMed]
- Komatsu S, Brustia R, Goumard C, et al. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: a matched pair analysis. Surg Endosc 2016;30:1965-74. [Crossref] [PubMed]
- Rhu J, Kim SJ, Choi GS, et al. Laparoscopic Versus Open Right Posterior Sectionectomy for Hepatocellular Carcinoma in a High-Volume Center: A Propensity Score Matched Analysis. World J Surg 2018;42:2930-7. [Crossref] [PubMed]
- Tarantino G, Magistri P, Serra V, et al. Laparoscopic Liver Resection of Right Posterior Segments for Hepatocellular Carcinoma on Cirrhosis. J Laparoendosc Adv Surg Tech A 2017;27:559-63. [Crossref] [PubMed]
- Xu HW, Liu F, Li HY, et al. Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc 2018;32:712-9. [Crossref] [PubMed]
- Yoon YI, Kim KH, Kang SH, et al. Pure Laparoscopic Versus Open Right Hepatectomy for Hepatocellular Carcinoma in Patients With Cirrhosis: A Propensity Score Matched Analysis. Ann Surg 2017;265:856-63. [Crossref] [PubMed]
- Zhang Y, Chen XM, Sun DL. Short-term Outcomes of Laparoscopic Versus Open Right Hemihepatectomy for Hepatocellular Carcinoma. Surg Laparosc Endosc Percutan Tech 2016;26:e157-e160. [Crossref] [PubMed]
- Zhang Y, Huang J, Chen XM, et al. A Comparison of Laparoscopic Versus Open Left Hemihepatectomy for Hepatocellular Carcinoma. Surg Laparosc Endosc Percutan Tech 2016;26:146-9. [Crossref] [PubMed]
- Takahara T, Wakabayashi G, Konno H, et al. Comparison of laparoscopic major hepatectomy with propensity score matched open cases from the National Clinical Database in Japan. J Hepatobiliary Pancreat Sci 2016;23:721-34. [Crossref] [PubMed]
- Palanisamy S, Sabnis SC, Patel ND, et al. Laparoscopic Major Hepatectomy-Technique and Outcomes. J Gastrointest Surg 2015;19:2215-22. [Crossref] [PubMed]
- Cauchy F, Fuks D, Nomi T, et al. Risk factors and consequences of conversion in laparoscopic major liver resection. Br J Surg 2015;102:785-95. [Crossref] [PubMed]
- Yang JH, Gu J, Dong P, et al. Isolated complete caudate lobectomy for hepatic tumor of the anterior transhepatic approach: surgical approaches and perioperative outcomes. World J Surg Oncol 2013;11:197. [Crossref] [PubMed]
- Cipriani F, Shelat VG, Rawashdeh M, et al. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J Am Coll Surg 2015;221:265-72. [Crossref] [PubMed]
- Farges O, Goutte N, Dokmak S, et al. How surgical technology translates into practice: the model of laparoscopic liver resections performed in France. Ann Surg 2014;260:916-21; discussion 921. [Crossref] [PubMed]
- Jia C, Li H, Wen N, et al. Laparoscopic liver resection: a review of current indications and surgical techniques. Hepatobiliary Surg Nutr 2018;7:277-88. [Crossref] [PubMed]
- Sotiropoulos GC, Prodromidou A, Machairas N. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma: The European experience. J BUON 2017;22:1160-71. [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Cho W, Kwon CHD, Choi JY, et al. Impact of technical innovation on surgical outcome of laparoscopic major liver resection: 10 years’ experience at a large-volume center. Ann Surg Treat Res 2019;96:14-8. [Crossref] [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Halls MC, Cipriani F, Berardi G, et al. Conversion for Unfavorable Intraoperative Events Results in Significantly Worse Outcomes During Laparoscopic Liver Resection: Lessons Learned From a Multicenter Review of 2861 Cases. Ann Surg 2018;268:1051-7. [Crossref] [PubMed]
- Morise Z, Ciria R, Cherqui D, et al. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci 2015;22:342-52. [Crossref] [PubMed]
- Komatsu S, Brustia R, Goumard C, et al. Clinical impact of laparoscopic hepatectomy: technical and oncological viewpoints. Surg Endosc 2017;31:1442-50. [Crossref] [PubMed]
- He YB, Bai L, Aji T, et al. Application of 3D reconstruction for surgical treatment of hepatic alveolar echinococcosis. World J Gastroenterol 2015;21:10200-7. [Crossref] [PubMed]
- Robu MR, Ramalhinho J, Thompson S, et al. Global rigid registration of CT to video in laparoscopic liver surgery. Int J Comput Assist Radiol Surg 2018;13:947-56. [Crossref] [PubMed]
- Nakashima Y, Nakashima O, Tanaka M, et al. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res 2003;26:142-7. [Crossref] [PubMed]
- Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007;245:36-43. [Crossref] [PubMed]
- Yoon SY, Kim KH, Jung DH, et al. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc 2015;29:2628-34. [Crossref] [PubMed]
- Cheung TT, Dai WC, Tsang SH, et al. Pure Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma in 110 Patients With Liver Cirrhosis: A Propensity Analysis at a Single Center. Ann Surg 2016;264:612-20. [Crossref] [PubMed]
- Chan FK, Cheng KC, Yeung YP, et al. Learning Curve for Laparoscopic Major Hepatectomy: Use of the Cumulative Sum Method. Surg Laparosc Endosc Percutan Tech 2016;26:e41-5. [Crossref] [PubMed]
- Brown KM, Geller DA. What is the Learning Curve for Laparoscopic Major Hepatectomy? J Gastrointest Surg 2016;20:1065-71. [Crossref] [PubMed]