
The miR-567/RPL15/TGF-β/Smad axis inhibits the stem-like properties and chemo-resistance of gastric cancer cells
Introduction
Gastric cancer (GC) is the second most significant contributor to cancer-related mortality in China, and has a high incidence in other East Asian countries such as Japan and Korea. (1,2). GC can mainly be attributed to genetic factors, high-salt foods, and helicobacter pylori infection (3-5). For patients at the advanced stage of the disease, the prognosis is poor, with a five-year survival rate that is usually very low (6,7). Cisplatin (DDP)-based chemotherapy is reported as the main strategy to prevent human gastric cancer (8,9). Therefore, it is of great importance that the pathogenesis of GC is explored so that the diagnosis and treatment of patients with the disease can be improved.
Several miRNAs including miR-424-5p, miR-125b, miR-21, and miR-17-92 are considered to be regulators of GC development and drug resistance (10). It has been reported that, through targeting FGF5, miR-567 suppresses the ability of osteosarcoma cells to proliferate, migrate, and invade (11). Furthermore, the miR-567 gene serves a role in tumor suppression as well as the inhibition of carcinogenesis in breast cancer (12). Studies have also shown that miR-567 inhibits GC tumorigenesis and chemoresistance through the miR-567-PIK3AP1-PI3K/AKT-c-Myc axis.
Cancer stem cells (CSCs), also known as cancer stem cells, are tumor cells with self-renewal and tumorigenic and metastatic properties (13), which were initially found in acute myeloid leukemia (14-16). The study of tumor stem cells was next extended to solid tumors, such as breast cancer (12), brain cancer (17), lung cancer (18), and colon cancer (19). Unlike tumor cells, tumor stem cells differentiate slowly on their own but are resistant to chemoradiotherapy. In the process of radiotherapy and chemotherapy, as long as the tumor stem cells exist, they will continue to differentiate into tumor cells, even if the tumor cells are killed (20). Therefore, reducing the stem-like characteristics of tumor cells has a positive effect on the treatment of tumors.
Ribosomal protein is a component of ribosome, which has a variety of secondary functions in DNA repair, apoptosis, drug resistance, and proliferation. Ribosomal protein L15 (RPL15) is one of the ribosomal proteins, which is upregulated in GC (21). Transforming growth factor β (TGF-β) family members are powerful negative regulators of mammalian skeletal muscle mass. The muscle mass of mice lacking TGF was found to be twice as high as that of normal mice (22). TGF binds to activin receptors and stimulates Smad2/3 signals to control the transcriptional activity of cell size-related genes (23). Previous studies have shown that activation of TGF-β/SMAD signaling pathway can induce EMT and promote GC transfer (24). However, the effect of miR-567 on the stem-like properties of GC cells and their potential molecular mechanism have not been explored. This study aimed to illuminate the role of the miR-567/RPL15/TGF-β/Smad axis in the regulation of the stem-like properties and chemo-resistance of GC cells.
Methods
Cell culture and spheroid body-forming
Gastric epithelial cells (GES-1) and GC cell lines (AGS, SCG-7901, MGC-803, SNU-16, and MKN1) were purchased from ATCC and stored in RPMI 1640 medium containing 10% FBS and antibiotics. Cell microsphere culture to obtain stemness was conducted with reference to Liu et al. (25). In brief, AGS cells (100 cells/well) were placed in serum-free RPMI-1640 medium supplemented with 1% N-2 supplement, 2% B-27 supplement (Invitrogen, Carlsbad, CA, USA) (Invitrogen), 1% antibiotic mixture (Invitrogen, Carlsbad, CA, USA), 20 ng/mL human FGF-2, and 100 ng/mL EGF (Chemicon). After 2 weeks, spheroid formation was analyzed and observed with an inverted microscope (Olym-pus, Tokyo, Japan) (Olympus) 40× magnification. Based on the method of Jiang et al. (26), the AGS cells were continuously exposed to cisplatin (from 0.05 to 1 mg/mL) to establish a cisplatin-resistant AGS cell (AGS/DDP) line.
Colony formation assay
AGS cells of control group, miR-567 group, LV-PRL15 group, miR-567 + LV-PRL15 group were pretreated with PBS, and were cultured in drug-free medium for approximate 14 days. The cells were fixed with cold methanol-glacial acetic acid and stained with crystal violet.
RT-qPCR
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from the GES-1, AGS, SCG-7901, MGC-803, SNU-16, and MKN1 cells according to the manufacturer’s instructions. cDNA was synthesized using PrimeScript RT kit (Takara Biotechnology, Dalian, China). qPCR was performed using SYBR Green Master Mix (Takara Biotechnology, Dalian, China) on a polymerase chain reaction (PCR) system. β-actin served as the endogenous control, and the data was normalized with β-actin and assessed using the 2-ΔΔCT method. The primers were synthesized by Invitrogen Company and the primer sequences used were as follows.
RPL15 Forward primer: 5'-CTGGGTTGGTGAAGATTCCA-3'
RPL15 Reverse forward 5'-GTGGACTGGTTTGGTGATCC-3'
Silencing of RPL15
PRL15-specific siRNA (si-PRL15) and negative control siRNA (siRNA NC) were designed and purchased from Invitrogen (USA). SiRNA-PRL15 or siRNA NC was transfected into AGS cells with Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. miR-567 mimic, miR-567 inhibitor, and matched negative controls (NC mimic and NC inhibitor) were synthesized by GenePharma (Shanghai, China). AGS cells were transfected using Lipofectamine 2000 (Invitrogen, Waltham, Beijing, China) and harvested after 48 h.
Luciferase assay
RPL15 3'UTR containing wild-type or mutant target site was amplified to construct RPL15 3'UTR (wt) and RPL15 3'UTR (mut) plasmids, respectively. AGS cells were transfected with NC or miR-567 mimics and RPL15 3'UTR (wt) or RPL15 3'UTR (mut). Luciferase activity was evaluated after 48 h.
Sphere formation assay
Around 1,000 AGS cells transfected with miR-567 mimic or LV-PRL15 or both were seeded into ultra-low-attachment 6-well plates in DMEM/F12 medium containing HEPES. Sphered colonies were observed under a microscope after 5 weeks.
Western blotting
Total protein was extracted from the cultivated cells with radioimmunoprecipitation assay solution (Thermo Fisher, Beijing, China). After denaturing, proteins samples were subjected to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The SDS-PAGE was then transferred to polyvinylidene fluoride (PVDF) membranes, followed by blocking for 2 hours at 25 °C in 5% non-fat milk. Subsequently, The PVDF membranes were sealed with 5% skimmed milk at 37 °C for 120 min and then, overnight incubated at 4 °C with the following primary antibodies: SOX2, NANOG, ALDH1A1, TGF-β1, TGFβ-R1, SMAD1, P-SMAD1, SMAD2, and P-SMAD2 (all Abcam, Beijing, China). Subsequently, PVDF membranes were incubated for 60 min at 37 °C with goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibodies (ab6721, 1:1,000, Abcam, Cambridge, MA, USA). After washing, signals were visualized using a ChemiDoc XRS imaging system and Quantity One analysis software (Bio-Rad, San Francisco, California, USA).
Cell viability assay
CCK 8 assay was conducted to measure AGS/DDP cell viability under exposure to cisplatin. About 3,000 cells were seeded into a single well of a 24-well plate and kept in an incubator at 37 °C with 5% CO2 for 48 h. Then, 10 µL of CCK-8 solution (Beyotime, Shanghai, China) was added to each well, before incubation for 2 h. The absorbance at 450 nm was detected by micro-plate reader (Bio-Rad, Hercules, CA, USA) and changes in cell viability were estimated.
EdU proliferation assay
Cell proliferation was detected by EdU proliferation assay. Three thousand cells were plated in 16-well chambered coverslips (Thermo Scientific, Waltham, MA, USA). After 48 h, the medium was changed to a 10 Mm 5-ethyny-2’-deoxyuridine (EdU) solution in FCS-free medium. After a period of 20 h, fixing was carried out with 3.7% formaldehyde. EdU incorporation was determined using the “EdU-Click 555” cell proliferation assay (Baseclick, Tutzing, Germany) in line with the instructions of the manufacturer. This assay the incorporation of EdU into DNA to be assessed by detection with fluorescing 5-carboxytetramethylrhodamine (5-TAMRA). Counterstaining of all nuclei was performed with DAPI, and cells were analyzed by fluorescence microscopy (excitation: 546 nm; emission: 479 nm).
Statistical analysis
Data are presented the mean ± SD based on three independent tests. Differences among groups were examined by one-way ANOVA analysis, and statistical significance was indicated by P<0.05.
Results
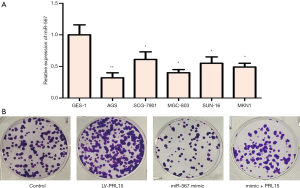
MiR-567 expression is low in GC cell lines
The expression of miR-567 in GES-1, AGS, SCG-7901, MGC-803, SUN-16, and MKN1 cell lines was detected by qPCR. The expression of miR-567 was downregulated in GC cell lines compared to normal gastric GES cells, while AGS cells had the lowest the expression level of miR-567 (Figure 1). Based on these findings, AGS was selected for further study.
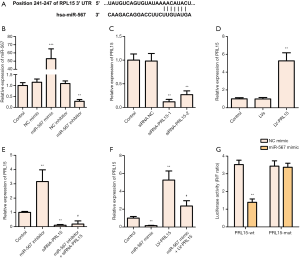
PRL15 is a direct target of miR-567
TargetScan analysis showed RPL15 to be a potential target for miR-567 (Figure 2A). After transfection with miR-567 mimic or miR-567 inhibitor, the level of miR-567 in AGS cells was significantly increased or decreased, respectively (Figure 2B). When ASC cells were transfected with si-PRL15, the level of PRL15 in cells was decreased significantly. When ASC cells were infected with lentivirus containing LV-PRL15, the level of PRL15 in cells was increased significantly (Figure 2C,D). Notably, low expression of miR-567 significantly upregulated the level of PRL15 in cells, while miR-567 overexpression had the opposite effect (Figure 2E,F). Double luciferase report assay showed that miR-567 overexpression significantly inhibited the expression of the luciferase gene by targeting PRL15, thus further confirming the relationship between miR-567 and PRL15 (Figure 2G). Taken together, these results show that PRL15 is a direct target of miR-567.
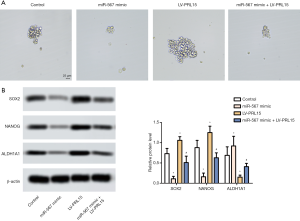
miR-567 regulates the stem cell-like properties of AGS cells
Sphere formation assay showed that when compared with the control group, the AGS cells transfected with miR-567 mimic formed smaller microspheres. AGS cells infected with lentivirus containing LV-PRL15 formed larger microspheres (Figure 3A). Western blotting analysis further discovered that miR-567 overexpression downregulated the expression of stem cell-like marker proteins (SOX2, NANOG, and ALDH1A1), while PRL15 overexpression exhibited the opposite effect (Figure 3B). All in all, these results showed that miR-567 regulates the stem cell-like properties of AGS cells.
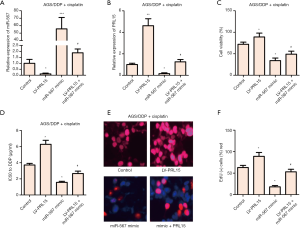
miR-567 enhances the sensitivity of AGS/DDP cells to cisplatin by targeting PRL15
AGS/DDP cells were transfected with LV-PRL15 or/and miR-567 mimic, respectively. The expression level of miR-567 was detected by RT-qPCR. The expression of PRL15 was significantly inhibited by the overexpression of miR-567 (Figure 4A). Further functional analysis showed that miR-567 overexpression inhibited the viability of AGS/DDP cells treated with cisplatin, while PRL15 overexpression had the opposite effect (Figure 4B). In addition, miR-567 overexpression decreased the IC50 value of AGS/DDP cells and inhibited cell proliferation (Figure 4C,D,E,F). PRL15 overexpression also exhibited the opposite effect. These results suggest that miR-567 enhances the sensitivity of AGS/DDP cells to cisplatin by targeting PRL15.
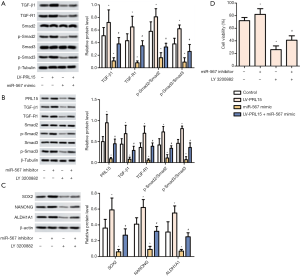
miR-567 reduces the stem-like properties and chemical sensitivity of GC cells by targeting PRL15 to regulate the TGF-β/Smad pathway
AGS cells were transfected with LV-PRL15 and/or miR-567 mimic. The protein levels of TGF-β1, TGF-R1, p-Smad2 and p-Smad3 were detected by Western blotting. The levels of TGF-β1, TGF β-R1, p-Smad2, and p-Smad3 were significantly upregulated by the overexpression of PRL15. In contrast, miR-567 overexpression had the opposite effect (Figure 5A). Furthermore, low expression of miR-567 promoted the expression of TGF-β1, TGF β-R1, p-Smad2, and p-Smad3. Notably, TGF-β/Smad pathway inhibitor LY 3200882 reversed the promoting effect of low miR-567 expression (Figure 5B). Further analysis revealed the low expression of miR-567 to promote the expression of stem-like marker proteins (SOX2, NANOG and ALDH1A1), and decrease the sensitivity of AGS/DDP cells to cisplatin (Figure 5C,D), while LY 3200882 showed the opposite effect. Taken together, these results suggest that miR-567 reduces the stem-like properties and chemical sensitivity of GC cells by targeting PRL15 to regulate the TGF-β/Smad pathway.
Discussion
GC is the second most significant contributor to cancer-related mortality in China. Environmental and genetic factors play a key role in the occurrence of GC (27,28). GC typically involves genetic changes, including the activation of oncogenes and the inactivation of tumor suppressor genes. GC stem-like cells (GCSCs), with unlimited self-renewal, differentiation, and tumor-regenerating capacities, contribute significantly to the refractory features of GC and have gained increasing attention for their role in GC drug resistance, relapse, and metastasis. CD44 was the first GCSC surface marker identified in GC cells, playing a critical role in tumor cell response to their microenvironment (29,30). In addition, these populations include the Villin+ and Lgr5+ GSCs in the antrum, the Troy+ chief cells in the corpus, and the Sox2+ GSCs that are found in both the antrum and the corpus. The recent identification of normal GSCs and gastric CSCs has greatly improved our understanding of the molecular and cellular etiology of GC and will aid in the development of effective therapies to treat patients (31). Importantly, miRNAs can promote either self-renewal or differentiation in stem cells, therefore, they are able to determine the fate of stem cells (32). Our research revealed the effect of miR-567 on the stem-like properties and chemical sensitivity of GC, while providing a new direction for the treatment of GC.
Previous studies have shown that miR-567 expression is downregulated in both GC tissue and GC cells (10). Our research supported this point and found that forced overexpression of miR-567 inhibited GC sphere formation. SOX2, NANOP, and ALDH1A1 are marker proteins of pluripotency and stem cell self-renewal (33,34), which are involved in tumorigenesis (35). In this study, the low expression of miR-567 was found to promote the expression of SOX2, NANOG, and ALDH1A1, and further increase the stem-like properties of AGS cells. In addition, overexpression of miR-567 enhanced the chemical sensitivity of AGS/DDP cells to cisplatin. Previous studies have shown that the mechanism of miR-567 lies in its direct targeting of PIK3A P1 to inactivate the PI3K/AKT/c-Myc pathway, thus regulating the formation and chemosensitivity of GC (10). On this basis, this study found that miR-567 reduced the stem-like properties and chemical sensitivity of GC cells to cisplatin by targeting PRL15, therefore establishing PRL15 as a direct target of miR-567.
Previous research have exhibited that RPL15 overexpression has been discovered in esophageal tumors (36). More importantly, in vitro and in vivo studies have revealed that low expression of RPL15 inhibits the proliferation of GC cells (21). In our study, we found that the overexpression of RPL15 enhanced the stem-like properties of AGS cells and reduced the sensitivity of AGS/DDP cells to cisplatin.
According to previous study, the activation of the TGF-β/Smad pathway increases the malignant degree of breast cancer (37), while in cervical cancer, through the regulation of the TGF-β/Smad pathway, the E7 oncogene has been shown to upregulate miR-182 expression (38). In addition, the involvement of the TGF-β/Smad signaling pathway in the proliferation and apoptosis of pancreatic cancer cells has also been proved (39). Conclusion, result in this research exhibited that low expression of miR-567 promotes the expression of the PRL15 protein as well as the activation of TGF-β/Smad pathway, therefore increasing the stem-like properties of AGS cells and decreasing the sensitivity of AGS/DDP cells to cisplatin.
Overall, this study established that miR-567 inhibits the stem-like properties and chemical resistance of GC cells by targeting RPL15 to inactivate the TGF-β/Smad pathway.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol 2006;12:17-20. [Crossref] [PubMed]
- Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol 2010;25:479-86. [Crossref] [PubMed]
- Ren JS, Kamangar F, Forman D, et al. Pickled food and risk of gastric cancer--a systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol Biomarkers Prev 2012;21:905-15. [Crossref] [PubMed]
- González CA, Sala N, Rokkas T. Gastric cancer: epidemiologic aspects. Helicobacter 2013;18:34-8. [Crossref] [PubMed]
- Forman D. Helicobacter pylori and Gastric Cancer. Scand J Gastroenterol Suppl 1996;215:48-51. [Crossref] [PubMed]
- Lu R, Chen Q, Liu X, et al. Detection of circulating stage III–IV gastric cancer tumor cells based on isolation by size of epithelial tumor: using the circulating tumor cell biopsy technology. Transl Cancer Res 2019;8:1342-50. [Crossref]
- Thrumurthy SG, Chaudry MA, Chau I, et al. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol 2015;12:676-82. [Crossref] [PubMed]
- Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009;20:666-73. [Crossref] [PubMed]
- Rennicke A, Voigt W, Mueller T, et al. Resistance mechanisms following cisplatin and oxaliplatin treatment of the human teratocarcinoma cell line 2102EP. Anticancer Res 2005;25:1147-55. [PubMed]
- Zhang F, Li K, Yao X, et al. A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer. EBioMedicine 2019;44:311-21. [Crossref] [PubMed]
- Liu D, Zhang C, Li X, et al. MicroRNA-567 inhibits cell proliferation, migration and invasion by targeting FGF5 in osteosarcoma. EXCLI J 2018;17:102-12. [PubMed]
- Bertoli G, Cava C, Diceglie C, et al. MicroRNA-567 dysregulation contributes to carcinogenesis of breast cancer, targeting tumor cell proliferation, and migration. Breast Cancer Res Treat 2017;161:605-16. [Crossref] [PubMed]
- Adorno-Cruz V, Kibria G, Liu X, et al. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res 2015;75:924-9. [Crossref] [PubMed]
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367:645-8. [Crossref] [PubMed]
- Mehrotra B, George TI, Kavanau K, et al. Cytogenetically aberrant cells in the stem cell compartment (CD34+lin-) in acute myeloid leukemia. Blood 1995;86:1139-47. [PubMed]
- Haase D, Feuring-Buske M, Konemann S, et al. Evidence for malignant transformation in acute myeloid leukemia at the level of early hematopoietic stem cells by cytogenetic analysis of CD34+ subpopulations. Blood 1995;86:2906-12. [Crossref] [PubMed]
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821-8. [PubMed]
- Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2008;15:504-14. [Crossref] [PubMed]
- Todaro M, Alea MP, Di Stefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 2007;1:389-402. [Crossref] [PubMed]
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756-60. [Crossref] [PubMed]
- Wang H, Zhao LN, Li KZ, et al. Overexpression of ribosomal protein L15 is associated with cell proliferation in gastric cancer. BMC Cancer 2006;6:91. [Crossref] [PubMed]
- Chen JL, Walton KL, Hagg A, et al. Specific targeting of TGF-beta family ligands demonstrates distinct roles in the regulation of muscle mass in health and disease. Proc Natl Acad Sci U S A 2017;114:E5266-5275. [PubMed]
- Winbanks CE, Chen JL, Qian H, et al. The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. J Cell Biol 2013;203:345-57. [Crossref] [PubMed]
- Zhang X, Zhang P, Shao M, et al. SALL4 activates TGF-beta/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag Res 2018;10:4459-70. [Crossref] [PubMed]
- Liu J, Ma L, Xu J, et al. Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int J Oncol 2013;42:453-9. [Crossref] [PubMed]
- Jiang T, Dong P, Li L, et al. MicroRNA-200c regulates cisplatin resistance by targeting ZEB2 in human gastric cancer cells. Oncol Rep 2017;38:151. [Crossref] [PubMed]
- Plummer M, Franceschi S, Munoz N. Epidemiology of gastric cancer. IARC Sci Publ 2004;157:311-26. [PubMed]
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 1999;49:33-64. [Crossref] [PubMed]
- Jafari N, Abediankenari S. MicroRNA-34 dysregulation in gastric cancer and gastric cancer stem cell. Tumour Biol 2017;39:1010428317701652. [Crossref] [PubMed]
- Fu Y, Du P, Zhao J, et al. Gastric Cancer Stem Cells: Mechanisms and Therapeutic Approaches. Yonsei Med J 2018;59:1150-8. [Crossref] [PubMed]
- Zhao Y, Feng F, Zhou YN. Stem cells in gastric cancer. World J Gastroenterol 2015;21:112-23. [Crossref] [PubMed]
- Wu Q, Yang Z, Wang F, et al. MiR-19b/20a/92a regulates the self-renewal and proliferation of gastric cancer stem cells. J Cell Sci 2013;126:4220-9. [Crossref] [PubMed]
- Chaudhary S, Islam Z, Mishra V, et al. A Regulatory Factor in Tumorigenesis and Metastasis. Current Protein & Peptide Science 2019;20:495-504. [Crossref] [PubMed]
- Boyer LA, Ihn LT, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005;122:947-56. [Crossref] [PubMed]
- Wang X, Ji X, Chen J, et al. SOX2 enhances the migration and invasion of ovarian cancer cells via Src kinase. PLoS One 2014;9:e99594. [Crossref] [PubMed]
- Wang Q, Yang C, Zhou J, et al. Cloning and characterization of full-length human ribosomal protein L15 cDNA which was overexpressed in esophageal cancer. Gene 2001;263:205-9. [Crossref] [PubMed]
- Dong H, Diao H, Zhao Y, et al. Overexpression of matrix metalloproteinase-9 in breast cancer cell lines remarkably increases the cell malignancy largely via activation of transforming growth factor beta/SMAD signalling. Cell Prolif 2019;52:e12633. [Crossref] [PubMed]
- Chen J, Deng Y, Ao L, et al. The high-risk HPV oncogene E7 upregulates miR-182 expression through the TGF-beta/Smad pathway in cervical cancer. Cancer Lett 2019;460:75-85. [Crossref] [PubMed]
- Chen G, Hu M, Wang XC, et al. Effects of RXRalpha on proliferation and apoptosis of pancreatic cancer cells through TGF-beta/Smad signaling pathway. Eur Rev Med Pharmacol Sci 2019;23:4723-9. [PubMed]


