Prognostic significance of the number of lymph nodes dissection in esophageal adenocarcinoma patients
Introduction
Esophageal cancer (EC) is one of the most common malignancies in the world (1). As reported by the 2018 Global Cancer Statistics, there are approximately 572,034 EC patients and 508,585 EC-related deaths worldwide (1). EC can be mainly classified as two types, namely, squamous cell carcinoma and adenocarcinoma. Among them, esophageal squamous cell carcinoma (ESCC) is the most common subtype of EC in the world, but the incidence of esophageal adenocarcinoma has increased sharply rapidly in numerous western countries over the past few decades, which even surpasses that the incidence of ESCC in the United Kingdom (UK), the Netherlands, Ireland, New Zealand, the United States (US), Australia, Denmark, Canada, and Sweden (2-4). Esophageal adenocarcinoma is considered as one of the fatal digestive tract malignancies, with the 5-year survival rate of as low as 16% (5). Esophagectomy combined with lymphadenectomy has long been adopted as the main treatment for EC, but there is no consensus on the number and scope of lymph node dissection (LND) among surgeons (6,7). A large number of relevant studies mainly involve ESCC, however, the relationship between the LND number and the prognosis for esophageal adenocarcinoma remains unclear and should be explained in further studies (8-11). Some researchers believe that, expanding the scope and number of LNDs contributes to removing more hidden positive lymph nodes and provides more accurate information on pathological staging, thus bringing superior prognosis for patients (11-13). Nonetheless, other researchers consider that, LND has limited benefit for EC patients; as a result, it is unnecessary to expand the score and number of LND, and they believe that this approach will not only bring more benefits to the patients, but also result in increased surgical risks and postoperative complications (7,12,14).
It remains controversial about whether more LNDs will lead to a longer survival, but most scholars generally believe that too few LNDs will not give rise to superior prognosis. In addition, no uniform conclusion is drawn concerning the minimal number of LND (14). In addition, it is noteworthy that most researchers do not apply different standards of LND scope and number for different tumor types. However, studies have shown that esophageal adenocarcinoma and ESCC possess different characteristics in lymph node metastasis (LNM) (15,16).
Therefore, this study aimed to analyze the relationship between LND number and the prognosis for esophageal adenocarcinoma patients based on the Surveillance, Epidemiology and End Results (SEER) database.
Methods
In this study, data were extracted from the SEER-18 registry of the US National Cancer Institute. Meanwhile, the SEER* Stat software version 8.3.6 was utilized to search and download data. Approval from the Institutional Review Board (IRB) was not needed since our data were extracted from a database.
Data selection
Relevant data were downloaded from all EC patients based on the SEER database from 2000 to 2016. Only non-metastatic primary EC was included in this study, while and excluded metastatic tumors from other sites were excluded. In the meantime, cases with incomplete survival information were also ruled out from this study. Patient treatment was limited to esophagectomy, and information on the number of LNDs should be included. Only esophageal adenocarcinoma patients were included in our study, and other cancer types were excluded. The following codes were classified as adenocarcinoma according to the International Classification of Diseases for Oncology third edition (ICD-O3), including 8140, 8144, 8210, 8261 and 8263.
The following information was also collected from those finally included cases, namely, age at diagnosis, gender, ethnicity, American Joint Committee on Cancer (AJCC) stage, tumor location, classification, the number of harvested lymph nodes, the number of harvested positive lymph nodes, tumor size, cause-specific death, vital status recoding, and survival (month).
For statistical analysis, the following continuous variables were transformed into categorical ones by according to age (<50, ≥50 years old group), the number of harvested lymph nodes (0, 1–10, 11–20, 21–30, >30 group), the number of harvested positive lymph nodes (0, 1–2, 3–4, 5–7, >7 group), and tumor size (0.1–1.0, 1.1–2.0, 2.1–3.0, 3.1–4.0, >4.0 cm group)
Statistical analysis
The SPSS 20 software was used for statistical analysis. Differences in categorical variables between groups were compared using χ2 test. The Kaplan-Meier product method was utilized for estimating the overall survival (OS) rate and disease-specific survival (DSS) among different LND groups. Afterwards, patients were stratified according to age, gender, grade, T stage and tumor size to analyze the relationship between the OS rate and the LND number. Besides, the Kaplan-Meier product method was utilized for estimation, whereas the log-rank test was used for comparison. The Cox proportional hazard model was employed to adjust the following confounding covariates, namely, age, gender, race, grade, T stage, tumor location, tumor size, and the number of positive nodes, so as to analyze the impact of LND number on OS and DSS after adjustment. All tests were two-sided, and a difference of P<0.05 were considered statistical significance.
Results
Patient characteristics
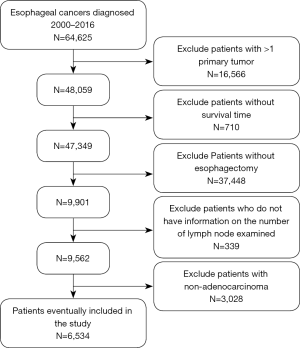
Data were obtained from 64,627 EC patients based on the SEER database, and 6,534 of them were finally included in our study according to the inclusion criteria. Among them, 8.9% patients had no lymph node harvested, 37.4% had 1–10 lymph nodes dissected, 34.1% had 11–20 lymph nodes harvested, 13.7% had 21–30 LND and 5.9% had over 30 lymph node harvested. Figure 1 shows the case selection process, and Table 1 presents the characteristics of each LND group.
Table 1
| Category | Number of lymph nodes dissected, no. (%) | P value | ||||
|---|---|---|---|---|---|---|
| 0 | 1–10 | 11–20 | 21–30 | >30 | ||
| Age (years old) | 0.242 | |||||
| <50 | 86 (14.7) | 307 (12.5) | 253 (11.4) | 103 (11.6) | 47 (12.2) | |
| ≥50 | 499 (85.3) | 2,140 (87.5) | 1,974 (88.6) | 786 (88.4) | 339 (87.8) | |
| Sex | 0.389 | |||||
| Male | 518 (88.5) | 2,199 (89.9) | 1,978 (88.8) | 787 (88.5) | 353 (91.5) | |
| Female | 67 (11.5) | 248 (10.1) | 249 (11.2) | 102 (11.5) | 33 (8.5) | |
| Race | 0.014 | |||||
| White | 563 (96.2) | 2,357 (96.3) | 2,132 (95.7) | 848 (95.4) | 359 (93.0) | |
| Black | 10 (1.7) | 44 (1.8) | 32 (1.4) | 16 (1.8) | 17 (4.4) | |
| Other | 11 (1.9) | 44 (1.8) | 59 (2.6) | 23 (2.6) | 8 (2.1) | |
| Unknown | 1 (0.2) | 2 (0.1) | 4 (0.2) | 2 (0.2) | 2 (0.5) | |
| Grade | <0.001 | |||||
| Grade I | 51 (8.7) | 187 (7.6) | 142 (6.4) | 49 (5.5) | 18 (4.7) | |
| Grade II | 227 (38.8) | 950 (38.8) | 861 (38.7) | 362 (40.7) | 165 (42.7) | |
| Grade III | 189 (32.3) | 1,023 (41.8) | 986 (44.3) | 382 (43.0) | 168 (43.5) | |
| Unknown | 118 (20.2) | 287 (11.7) | 238 (10.7) | 96 (10.8) | 35 (9.1) | |
| T stage | <0.001 | |||||
| T1 | 147 (25.1) | 528 (21.6) | 454 (20.4) | 177 (19.9) | 72 (18.7) | |
| T2 | 51 (8.7) | 258 (10.5) | 257 (11.5) | 102 (11.5) | 53 (13.7) | |
| T3 | 133 (22.7) | 781 (31.9) | 875 (39.3) | 390 (43.9) | 164 (42.5) | |
| T4 | 23 (2.9) | 89 (3.6) | 87 (3.9) | 43 (4.8) | 15 (3.9) | |
| Unknown | 231 (39.5) | 791 (32.3) | 554 (24.9) | 177 (19.9) | 82 (21.2) | |
| Tumor location | 0.028 | |||||
| Upper third | 6 (1.0) | 16 (0.7) | 11 (0.5) | 5 (0.6) | 3 (0.8) | |
| Middle third | 30 (5.1) | 124 (5.1) | 92 (4.1) | 43 (4.8) | 20 (5.2) | |
| Lower third | 480 (82.1) | 2,122 (86.7) | 1,955 (87.8) | 786 (88.4) | 337 (87.3) | |
| Unknown | 69 (11.8) | 185 (7.6) | 169 (7.6) | 55 (6.2) | 26 (6.7) | |
| Tumor size, cm | <0.001 | |||||
| 0.1–1.0 | 31 (5.3) | 158 (6.5) | 125 (5.6) | 54 (6.1) | 27 (7.0) | |
| 1.1–2.0 | 23 (3.9) | 205 (8.4) | 215 (9.7) | 66 (7.4) | 28 (7.3) | |
| 2.1–3.0 | 39 (6.7) | 225 (9.2) | 240 (10.8) | 112 (12.6) | 43 (11.1) | |
| 3.1–4.0 | 39 (6.7) | 221 (9.0) | 224 (10.1) | 91 (10.2) | 38 (9.8) | |
| >4.0 | 83 (14.2) | 477 (19.5) | 543 (24.4) | 261 (29.4) | 106 (27.5) | |
| Unknown | 370 (63.2) | 1,161 (47.4) | 880 (39.5) | 305 (34.3) | 144 (37.3) | |
Multivariate analysis for OS and DSS
According to the multivariate analysis results, the <50 (vs. >50 years old), women (vs. men), Grade I (vs. Grade III), T1 (vs. T2, T3), smaller tumor, no positive lymph node, and the number of harvested lymph nodes in 21–30 and >30 (vs. no harvested lymph node group) groups were associated with higher OS and DSS (Table 2). Moreover, results on the LND number suggested no statistical difference in OS and DSS for 0, 1–10 group [OS: hazard ratio (HR): 0.94, 95% confidence interval (CI): 0.559–1.58; DSS: HR: 0.934, 95% CI: 0.522–1.669], and 11–20 group (OS: HR: 0.693, 95% CI: 0.412–1.165; DSS: HR: 0.706, 95% CI: 0.395–1.262). In addition, the 21–30 group (OS: HR: 0.546, 95% CI: 0.323–0.924; DSS: HR: 0.541, 95% CI: 0.301–0.973) and >30 group (OS: HR: 0.542, 95% CI: 0.318–0.923; DSS: HR: 0.517, 95% CI: 0.285–0.939) had better OS and DSS compared with those of 0 group.
Table 2
| Category | No. (%) of patients | OS | DSS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years old) | ||||||
| <50 | 796 (12.2) | Reference | Reference | |||
| ≥50 | 5,738 (87.5) | 1.391 (1.258–1.538) | <0.001 | 1.23 (1.106–1.367) | <0.001 | |
| Sex | ||||||
| Male | 5,835 (89.3) | Reference | Reference | |||
| Female | 699 (10.7) | 0.836 (0.751–0.93) | 0.001 | 0.819 (0.727–0.923) | 0.001 | |
| Race | ||||||
| White | 6,259 (95.8) | Reference | Reference | |||
| Black | 119 (1.8) | 0.942 (0.735–1.207) | 0.636 | 0.939 (0.717–1.23) | 0.648 | |
| Other | 145 (2.2) | 0.906 (0.71–1.156) | 0.426 | 0.908 (0.697–1.184) | 0.478 | |
| Unknown | 11 (0.2) | |||||
| Grade | ||||||
| Grade I | 447 (6.8) | Reference | Reference | |||
| Grade II | 2,565 (39.3) | 1.146 (0.99–1.328) | 0.068 | 1.192 (1.004–1.417) | 0.045 | |
| Grade III | 2,748 (42.1) | 1.491 (1.288–1.726) | <0.001 | 1.638 (1.381–1.944) | <0.001 | |
| Unknown | 774 (11.8) | |||||
| T stage | ||||||
| T1 | 1,378 (21.1) | Reference | Reference | |||
| T2 | 721 (11.0) | 1.401 (1.225–1.602) | <0.001 | 1.541 (1.324–1.793) | <0.001 | |
| T3 | 2,343 (35.9) | 1.821 (1.632–2.032) | <0.001 | 2.002 (1.766–2.27) | <0.001 | |
| T4 | 257 (3.9) | 1.795 (1.504–2.143) | <0.001 | 1.929 (1.586–2.345) | <0.001 | |
| Unknown | 1,835 (28.1) | |||||
| Tumor location | ||||||
| Upper third | 41 (0.6) | Reference | Reference | |||
| Middle third | 309 (4.7) | 1.49 (0.929–2.391) | 0.098 | 1.489 (0.876–2.531) | 0.142 | |
| Lower third | 5,680 (86.9) | 1.174 (0.747–1.844) | 0.487 | 1.131 (0.68–1.881) | 0.635 | |
| Unknown | 504 (7.7) | |||||
| Tumor size, cm | ||||||
| 0.1–1.0 | 395 (6.0) | Reference | Reference | |||
| 1.1–2.0 | 537 (8.2) | 1.274 (1.033–1.569) | 0.023 | 1.407 (1.094–1.81) | 0.008 | |
| 2.1–3.0 | 659 (10.1) | 1.348 (1.102–1.65) | 0.004 | 1.553 (1.219–1.977) | <0.001 | |
| 3.1–4.0 | 613 (9.4) | 1.401 (1.142–1.72) | 0.001 | 1.624 (1.273–2.072) | <0.001 | |
| >4.0 | 1,470 (22.5) | 1.352 (1.116–1.638) | 0.002 | 1.592 (1.264–2.005) | <0.001 | |
| Unknown | 2,860 (43.8) | |||||
| Number of nodes resected | ||||||
| 0 | 585 (9.0) | Reference | Reference | |||
| 1–10 | 2,447 (37.5) | 0.94 (0.559–1.58) | 0.815 | 0.934 (0.522–1.669) | 0.817 | |
| 11–20 | 2,227 (34.1) | 0.693 (0.412–1.165) | 0.166 | 0.706 (0.395–1.262) | 0.24 | |
| 21–30 | 889 (13.6) | 0.546 (0.323–0.924) | 0.024 | 0.541 (0.301–0.973) | 0.04 | |
| >30 | 386 (5.9) | 0.542 (0.318–0.923) | 0.024 | 0.517 (0.285–0.939) | 0.03 | |
| Number of nodes positive | ||||||
| 0 | 3,535 (54.1) | Reference | Reference | |||
| 1–2 | 1,237 (18.9) | 1.718 (1.578–1.871) | <0.001 | 1.922 (1.751–2.111) | <0.001 | |
| 3–4 | 510 (7.8) | 2.601 (2.326–2.908) | <0.001 | 2.986 (2.649–3.365) | <0.001 | |
| 5–7 | 324 (5.0) | 2.903 (2.546–3.312) | <0.001 | 3.352 (2.915–3.854) | <0.001 | |
| >7 | 311 (4.8) | 4.983 (4.346–5.714) | <0.001 | 5.769 (4.994–6.664) | <0.001 | |
| Unknown | 617 (9.4) | |||||
OS, overall survival; DSS, disease-specific survival; HR, hazard ratio; CI, confidence interval.
Survival outcomes and subgroup analysis
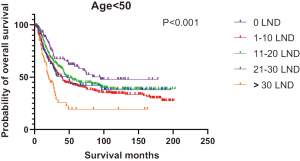
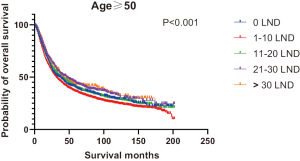
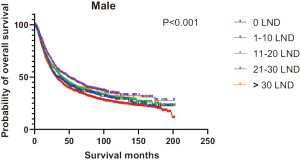
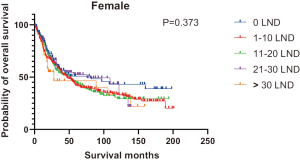
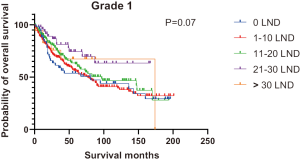
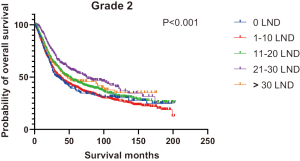
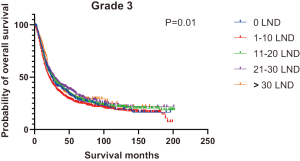
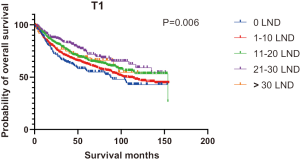
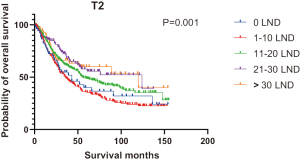
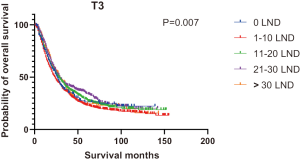
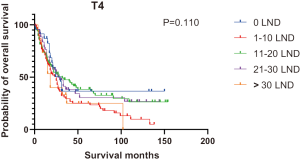
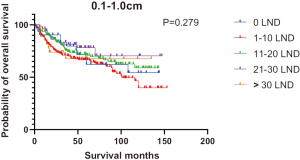
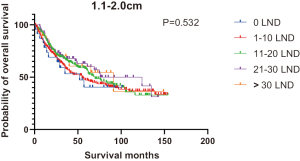
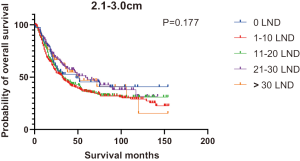
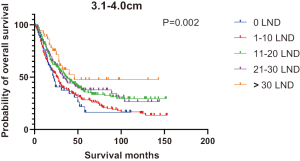
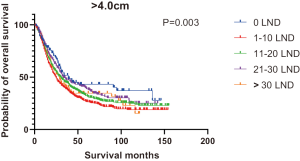
Figures 2,3 presents the Kaplan-Meier curve of OS and DSS for different LND number groups. Table 3 summarizes the results of subgroup analysis, and the Kaplan-Meier curve of OS for each subgroup is summarized in Figures S1-S16. According to the age-stratified subgroup analysis, there were statistically significant differences in the number of LND between <50 and ≥50 years old groups. Typically, the 21–30 lymph nodes resected group had the longest median survival (95 months) in <50 years old Group, while the >30 lymph nodes resected group had the shortest median survival (20 months). In the ≥50 years old group, the 21–30 lymph nodes resected group had the longest median survival (46 months), and the 1–10 lymph nodes resected group had the shortest median survival (31 months). In gender-stratified subgroup analysis, statistical differences were only detected in the male subgroup, and the 21–30 lymph nodes resected group had the longest median survival (49 months), while the 1–10 lymph nodes resected group had the shortest median survival (31 months). In Grade-stratified subgroup analysis, there were statistical differences in Grade I, Grade II and Grade III subgroups. In the Grade I subgroup, the 21–30 lymph nodes resected group had the longest median survival, and 1–10 lymph nodes resected group had the shortest median survival (73 months). In Grade II subgroup, the 21–30 lymph nodes resected group had the longest median survival (75 months), while the 0 lymph node resected group had has the shortest median survival (32 months). In Grade III subgroup, both the 21–30 lymph nodes resected group and the >30 lymph nodes resected group had the longest median survival (29 months), and the 1–10 node resected group has the shortest median survival (21 months). In T stage-stratified subgroup analysis, there were statistical differences in T1, T2, and T3 subgroups. In the T1 subgroup, 21–30 nodes resected group has the longest median survival, while the 0 lymph node resected group showed the shortest median survival (88 months). In the T2 subgroup, the 21–30 lymph nodes resected group and the >30 lymph nodes resected group had the longest median survival (124 months), whereas the 1–10 lymph nodes resected group displayed the shortest median survival (36 months). In the T3 subgroup, both the 21–30 lymph nodes resected group and the >30 lymph nodes resected group exhibited the longest median survival (29 months), while the 1–10 lymph nodes resected group had the shortest median survival (23 months). In the tumor size-stratified subgroup analysis, differences in 3.1–4.0 and >4 cm. In the tumor size of 3.1–4.0 cm subgroup, the >30 lymph nodes resected group showed the longest median survival (46 months), whereas the 0 lymph node resected group had the shortest median survival (22 months). In the tumor size of >4 cm subgroup, the 0 lymph node resected group displayed the longest median survival (39 months), while the 1–10 lymph nodes resected group had the shortest median survival (24 months).
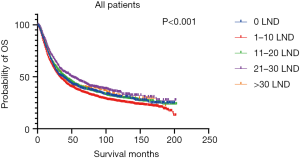
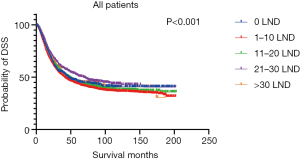
Table 3
| Category | Median survival time (months) | P value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1–10 | 11–20 | 21–30 | >30 | Total | ||
| Age (years old) | |||||||
| <50 | 47 | 39 | 49 | 95 | 20 | 43 | <0.001 |
| ≥50 | 35 | 31 | 38 | 46 | 40 | 36 | <0.001 |
| Sex | |||||||
| Male | 32 | 31 | 39 | 49 | 38 | 36 | <0.001 |
| Female | 82 | 47 | 51 | 76 | 28 | 50 | 0.373 |
| Grade | |||||||
| Grade I | 81 | 73 | 83 | NA | 174 | 84 | 0.07 |
| Grade II | 32 | 38 | 49 | 75 | 45 | 44 | <0.001 |
| Grade III | 24 | 21 | 26 | 29 | 29 | 24 | 0.01 |
| T stage | |||||||
| T1 | 88 | 102 | 154 | NA | NA | 120 | 0.006 |
| T2 | 43 | 36 | 55 | 124 | 120 | 51 | 0.001 |
| T3 | 26 | 23 | 28 | 29 | 29 | 26 | 0.007 |
| T4 | 30 | 23 | 28 | 27 | 18 | 25 | 0.110 |
| Tumor size, cm | |||||||
| 0.1–1.0 | NA | 101 | NA | NA | NA | NA | 0.279 |
| 1.1–2.0 | 52 | 55 | 70 | 124 | 91 | 65 | 0.532 |
| 2.1–3.0 | 48 | 32 | 32 | 59 | 52 | 36 | 0.177 |
| 3.1–4.0 | 22 | 25 | 37 | 33 | 46 | 30 | 0.002 |
| >4.0 | 39 | 24 | 30 | 33 | 28 | 29 | 0.003 |
NA, not available.
Discussion
Our results indicated that, the number of LND was an independent prognostic factor for OS and DSS, and that the LND number of >20 was associated with superior OS and DSS. It was also found in subgroup analysis that, differences in the OS for different LND groups was statistically significant in <50, ≥50 years old, male, Grade I, Grade II, Grade III, T1, T2, T3 and tumor size >4 cm subgroup. Our results were similar to previous studies, indicating the importance of LND to prognosis (17-22). It has been discovered for several centuries that, LNM is associated with the poor prognosis for cancer patients (23,24). Afterwards, a large number of scholars have continuously explored the specific mechanism of LNM in the poor prognosis and the theoretical basis of LND. Some evidence proves that tumor cells metastasizing from lymphatic vessels to lymph nodes can enter the blood circulation through a thoracic catheter (23). In addition, two studies using tumor-bearing mouse models show that, the metastatic tumor cells in sentinel lymph nodes can enter the lymph node blood vessels and spread to distant organs (25,26). Therefore, the existing evidence proves that, it is of great significance to remove lymph nodes with occult metastasis to reduce the risk of distant metastasis and improve patient prognosis.
The appropriate number and scope of LND have always been controversial among surgeons, and numerous studies have been conducted to confirm their conclusions. Notably, three- and two-field LNDs are currently the two most well-recognized approaches. Compared with two-field LND, three-field LND has added cervical LND (12,27). A large number of retrospective studies are conducted to examine the survival rate, but no consistent conclusion is reached at present (9). Nonetheless, it is proved in two randomized controlled trials (RCTs) that, three-field LND can provide better survival results (28,29). Additionally, two meta-analyses of retrospective studies have reached the same conclusion, suggesting that a wider LND scope often indicates a larger number of lymph nodes harvested, a higher possibility of removing the occult lymph nodes, and an important role in the accurate staging of lymph nodes (9,30). Noteworthily, not all the number of lymph nodes harvested plays a role. As figured out from our results, a too low number of LND did not improve the prognosis for esophageal adenocarcinoma patients, and only the LND number of over 20 was effective. However, there are no unified conclusions on the suitable scope of LND among EC patients from different studies. Yuan et al. showed in their study on ESCC that, at least 29 lymph nodes should be removed to maximize the postoperative survival (31). Groth et al. analyzed based on the SEER database and discovered that the dissection of over 12 lymph nodes improved patient outcomes, and that the risk of death significantly reduced when over 30 lymph nodes were dissected (18). Additionally, Almhanna et al. from a tertiary cancer center demonstrated that, only the LND number of 13–20 improved patient prognosis (32). All the above-mentioned studies suggest that, only a sufficient number of LNDs improves patient prognosis, and there is still no uniform conclusion on the optimal scope of dissection; in particular, there is even scarce related research on esophageal adenocarcinoma. Therefore, more high-quality studies are warranted to determine the optimal scope of LND.
Until now, a large number of studies on the scope and number of LND in EC do not treat adenocarcinoma in a different way from squamous cell carcinoma (9,30). However, some studies reveal significant differences in LNM between esophageal adenocarcinoma and ESCC. According to a propensity matching study by Deng et al., esophageal adenocarcinoma was associated with a higher number of positive lymph nodes and a higher rate of LNM than those of EC (15). Besides, Rice et al. showed that esophageal adenocarcinoma was more prone to LNM than ESCC (16). Therefore, it is necessary to explore the surgical methods to expand or reduce the scope and number of LND according to the LNM characteristics of different tumor types.
Apart from the number of LNM that can serve as the prognostic factor for patients with esophageal adenocarcinoma, our results also showed that age, gender, differentiation degree, T stage, tumor size, and number of positive lymph nodes were also the prognostic factors. In addition, multiple studies indicate that, tumor response to neoadjuvant chemotherapy or radiochemotherapy, the patient performance status, co-morbidities, and health-related quality of life can also serve as the prognostic factors for esophageal adenocarcinoma patients (2).
At present, researchers still have diverse views on the treatment for esophageal adenocarcinoma, but the current major treatment strategies are quite similar. Among them, the endoscopic treatment for early esophageal adenocarcinoma has gradually become the mainstream treatment (33). However, EC at T1sm2–3 stage is excluded from endoscopic treatment, since it is associated with significantly higher risk of LNM than that at T1sm1 stage (33), as proved by our results from T1 stage subgroup analysis. LND affects the prognosis for T1 stage patients; therefore, it is necessary to further refine the treatment strategies according to the depth of tumor invasion. For patients with locally advanced esophageal adenocarcinoma, surgery remains the preferred treatment (5). Compared with patients treated with surgery alone, those receiving combined perioperative adjuvant therapy have superior prognosis (5,34). For patients who are deprived of the chance of surgery or can not tolerate surgery, radiotherapy and chemotherapy are the definite treatment options. Meanwhile, some evidence proves that, the multimodal therapy of radiotherapy and chemotherapy seems to result in superior prognosis (5).
Nonetheless, certain limitations should be noted in this study. First of all, there was inevitable selection bias in this study due to its retrospective nature. Secondly, information on comorbidities, pulmonary function, perioperative radiotherapy and chemotherapy, surgical procedure, and specific LND areas is lacking in the database, so they were not incorporated in multivariate analysis.
Conclusions
To sum up, the number of LND serves as an independent prognostic factor for OS and DSS in patients with esophageal adenocarcinoma. In addition, we recommend that esophageal adenocarcinoma patients should undergo LND to dissect at least 20 lymph nodes.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2802). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval from the Institutional Review Board (IRB) was not needed since our data were extracted from a database.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Coleman HG, Xie SH, Lagergren J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018;154:390-405. [Crossref] [PubMed]
- Lam AK. Introduction: esophageal adenocarcinoma: updates of current status. Methods Mol Biol 2018;1756:1-6.
- McColl KEL. What is causing the rising incidence of esophageal adenocarcinoma in the West and will it also happen in the East? J Gastroenterol 2019;54:669-73. [Crossref] [PubMed]
- Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology 2015;149:302-17.e1. [Crossref] [PubMed]
- Takahashi C, Shridhar R, Huston J, et al. Esophagectomy from then to now. J Gastrointest Oncol 2018;9:903-9. [Crossref] [PubMed]
- Yeung JC, Bains MS, Barbetta A, et al. How many nodes need to be removed to make esophagectomy an adequate cancer operation, and does the number change when a patient has chemoradiotherapy before surgery? Ann Surg Oncol 2020;27:1227-32. [Crossref] [PubMed]
- Lin Z, Chen W, Chen Y, et al. Achieving adequate lymph node dissection in treating esophageal squamous cell carcinomas by radical lymphadenectomy: Beyond the scope of numbers of harvested lymph nodes. Oncol Lett 2019;18:1617-30. [PubMed]
- Ma GW, Situ DR, Ma QL, et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol 2014;20:18022-30. [Crossref] [PubMed]
- Zhao Y, Mao Y. Pattern of lymph node metastasis and choice of lymphadenectomy in patients with thoracic esophageal squamous cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:987-94. [PubMed]
- Fan N, Yang H, Zheng J, et al. Comparison of short- and long-term outcomes between 3-field and modern 2-field lymph node dissections for thoracic oesophageal squamous cell carcinoma: a propensity score matching analysis. Interact Cardiovasc Thorac Surg 2019;29:434-41. [Crossref] [PubMed]
- Shang QX, Chen LQ, Hu WP, et al. Three-field lymph node dissection in treating the esophageal cancer. J Thorac Dis 2016;8:E1136-49. [Crossref] [PubMed]
- Yu L, Zhang XT, Guan SH, et al. The number of negative lymph nodes is positively associated with survival in esophageal squamous cell carcinoma patients in China. Open Med (Wars) 2020;15:152-9. [Crossref] [PubMed]
- Zhan C, Shi Y, Jiang W, et al. How many lymph nodes should be dissected in esophagectomy with or without neoadjuvant therapy to get accurate staging? Dis Esophagus 2020; [Crossref] [PubMed]
- Deng HY, Wang ZQ, Wang YC, et al. Oesophageal adenocarcinoma has a higher risk of lymph node metastasis than squamous cell carcinoma: a propensity score-matched study. Eur J Cardiothorac Surg 2017;52:958-62. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Hofstetter WL, et al. Esophageal Cancer: Associations With (pN+) Lymph Node Metastases. Ann Surg 2017;265:122-9. [Crossref] [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Groth SS, Virnig BA, Whitson BA, et al. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: data from the Surveillance Epidemiology and End Results database. J Thorac Cardiovasc Surg 2010;139:612-20. [Crossref] [PubMed]
- Wu SG, Zhang ZQ, Liu WM, et al. Impact of the number of resected lymph nodes on survival after preoperative radiotherapy for esophageal cancer. Oncotarget 2016;7:22497-507. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Xia W, Liu S, Mao Q, et al. Effect of lymph node examined count on accurate staging and survival of resected esophageal cancer. Thorac Cancer 2019;10:1149-57. [Crossref] [PubMed]
- Liu Y, Yang H, Fu H, et al. Prognostic impact of examined lymph node count in pT1N0M0 esophageal cancer: a population-based study. Thorac Cancer 2019;10:1636-43. [Crossref] [PubMed]
- Farnsworth RH, Achen MG, Stacker SA. The evolving role of lymphatics in cancer metastasis. Curr Opin Immunol 2018;53:64-73. [Crossref] [PubMed]
- Ma Q, Dieterich LC, Detmar M. Multiple roles of lymphatic vessels in tumor progression. Curr Opin Immunol 2018;53:7-12. [Crossref] [PubMed]
- Brown M, Assen FP, Leithner A, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018;359:1408-11. [Crossref] [PubMed]
- Pereira ER, Kedrin D, Seano G, et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 2018;359:1403-7. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Three-field lymph node dissection in esophageal cancer surgery. J Thorac Dis 2017;9:S731-40. [Crossref] [PubMed]
- Isono K, Onoda S, Ishikawa T, et al. Studies on the causes of deaths from esophageal carcinoma. Cancer 1982;49:2173-9. [Crossref] [PubMed]
- Kato H, Watanabe H, Tachimori Y, et al. Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. Ann Thorac Surg 1991;51:931-5. [Crossref] [PubMed]
- Ye T, Sun Y, Zhang Y, et al. Three-field or two-field resection for thoracic esophageal cancer: a meta-analysis. Ann Thorac Surg 2013;96:1933-41. [Crossref] [PubMed]
- Yuan F, Qingfeng Z, Jia W, et al. Influence of metastatic status and number of removed lymph nodes on survival of patients with squamous esophageal carcinoma. Medicine (Baltimore) 2015;94:e1973. [Crossref] [PubMed]
- Almhanna K, Weber J, Shridhar R, et al. Determining the optimal number of lymph nodes harvested during esophagectomy. J Gastrointest Oncol 2016;7:387-94. [Crossref] [PubMed]
- Hammoud GM, Hammad H, Ibdah JA. Endoscopic assessment and management of early esophageal adenocarcinoma. World J Gastrointest Oncol 2014;6:275-88. [Crossref] [PubMed]
- Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 2020;50:12-20. [Crossref] [PubMed]