
Claudin 18.2 expression in various tumor types and its role as a potential target in advanced gastric cancer
Introduction
Tight junctions are specialized membrane domains at the most apical region of polarized epithelial and endothelial cells (1). Claudins, crucial components of tight junctions, are transmembrane proteins with extracellular loops, which are potential targets for diagnostic and therapeutic modalities (2-4). They may play an important role in tumorigenesis and inflammation. Alterations in claudin expression have been shown to lead to impaired functions of tight junctions, influence signaling pathways, and act as tumor promoting events in some epithelial cancers (3-6). In tumors, tight junctions become disrupted and claudin proteins lose their primary role. Claudins are abnormally controlled in various cancers, including gastric cancer (GC), hepatocellular carcinoma (HCC), biliary tract cancer (BTC), breast cancer, renal cell carcinoma, pancreatic cancer (PC), non-small cell lung cancer, and mesothelioma (7-15).
Claudin 18.2, a member of the claudin family, is commonly expressed in multiple cancers, including GC and PC (9,15,16). Claudin 18.2 is not expressed in any healthy tissues with the exception of gastric mucosa. Recently, zolbetuximab, a highly potent chimeric IgG1 mAb that binds to claudin 18.2 on the surface of tumour cells, was developed and investigated in clinical trials (9,17). Notably, in a phase II trial, zolbetuximab with standard chemotherapy as a first-line treatment improved the median survival in claudin 18.2-expressing patients with GC compared to chemotherapy alone (NCT01630083) (18). This promising result suggests that clinical trials using this novel agent are needed to extend its clinical application to other cancer types (19,20). Considering that patients positive for human epidermal growth factor receptor 2 (HER2), who are currently been treated with the novel agent trastuzumab, comprise only 10–15% of all incidences of GC (21), the broad expression of claudin 18.2 is an important and remarkable finding for cancer treatment.
Importantly, the claudin 18.2 status across different tumor types has not been well studied using immunohistochemistry (IHC). To investigate the role of claudin 18.2 as a biomarker, we conducted a prospective claudin 18.2 IHC study in a cohort of patients with various solid cancer tumors.
Methods
Ethics
The study was approved by the institutional review board of the Samsung Medical Center (Seoul, Korea) (No. 2013-10-017), and all patients provided written informed consent before enrollment. This study was conducted in accordance with the Declaration of Helsinki.
Patients
Patients (n=430) with various solid cancer tumors were evaluated for claudin 18.2 expression from June 2012 to March 2016 at the Samsung Medical Center. Enrolled patients provided written informed consent before study entry. The clinicopathological characteristics for all enrolled patients were reviewed.
IHC of claudin 18
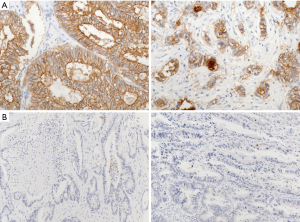
Representative tumor lesions were chosen, and a tissue microarray was constructed after review of a hematoxylin and eosin-stained section from the block. Two representative regions of the tumor were then sampled from the donor block. Cores of 2-mm diameter were extracted and embedded in the array block. Tumor sections from array blocks were freshly cut to 3 µm and dried at 60 °C for 30 minutes. Claudin 18 IHC was carried out using a BOND-MAX autoimmunostainer (Leica Microsystems, Wetzlar, Germany) with BOND Polymer Refine Detection (DS9800; Vision BioSystems, Melbourne, Australia) according to the manufacturer’s protocol. Briefly, the slides were deparaffinized and incubated for 20 minutes with buffer (pH 6.0) in 97 °C and endogenous peroxidase blocking solution for 5 minutes. A claudin 18 rabbit polyclonal antibody (Thermo Fisher Scientific, Carlsbad, CA, USA), diluted 1:150, was used as the primary antibody, and samples were incubated for 15 minutes at room temperature. Claudin 18 expression was assessed based on the intensity of the membrane staining, and the IHC was interpreted as positive when a weak membrane staining was visible in >5% of tumor cells. Representative positive and negative examples are shown in Figure 1.
Statistics
Descriptive statistics are presented as proportions and medians. Data are also shown as number (%) for categorical variables. Correlation of the status of claudin 18.2 and clinicopathologic features was evaluated with the t-test or the Fisher’s exact test, as appropriate, or one-way analysis of variance (ANOVA). Kaplan-Meier estimates were used in the analysis of all time-to event variables, and the 95% confidence interval (CI) for the median time to event was computed.
Results
Patient characteristics
The clinicopathologic features of all 430 patients are shown in Table 1. The most frequent tumor type was gastrointestinal cancer. Colorectal cancer (CRC) (n=203, 47.2%), and GC (n=85, 19.8%) were common. Most patients (78.6%, 338/430) presented with stage IV disease and 173 patients with stage IV disease (51.2%) had two or more metastatic lesions.
Table 1
| Clinicopathologic variable | Sample size, n (%) |
|---|---|
| Gender | |
| Male | 249 (57.9) |
| Female | 181 (42.1) |
| Age | |
| Median (range) | 59.0 (19.0–89.0) |
| ≤65 | 303 (70.5) |
| >65 | 127 (29.5) |
| Tumor type | |
| Gastric cancer (GC) | 85 (19.8) |
| Colorectal cancer (CRC) | 203 (47.2) |
| Genitourinary (GU) tract cancer | 46 (10.7) |
| Biliary tract cancer (BTC) | 16 (3.7) |
| Pancreatic cancer (PC) | 6 (1.4) |
| Sarcoma | 37 (8.6) |
| Melanoma | 8 (1.9) |
| Hepatocellular carcinoma (HCC) | 15 (3.5) |
| Miscellaneous | 14 (3.3) |
| Disease extent | |
| Locally advanced disease | 92 (21.4) |
| Metastatic disease | 338 (78.6) |
Claudin 18.2 expression by tumor type
Nearly all patients (414/430, 96.3%) were included in the claudin 18.2 expression study using IHC. Irrespective of the tumor type, 4.1% (17/414) were claudin 18.2-positive according to a weak membrane staining in >5% of tumor cells. Claudin 18.2 expression by tumor type is shown in Table 2. It was positive in 16.7% of patients with PC, 14.1% of those with GC, 6.3% of those with BTC, 2.2% of those with genitourinary (GU) cancer/miscellaneous tumors, and 0.9% of those with CRC. Representative images of claudin 18.2 IHC staining are shown in Figure 1.
Table 2
| Tumor type | Total (n=430) | Claudin 18.2+, n (%) | Claudin 18.2−, n (%) | Non-evaluable, n (%) |
|---|---|---|---|---|
| Gastric cancer (GC) | 85 | 12 (14.1) | 72 (84.7) | 4 (4.7) |
| Colorectal cancer (CRC) | 203 | 2 (0.9) | 195 (96.1) | 6 (3.0) |
| Genitourinary tract cancers (GU) | 46 | 1 (2.2) | 42 (91.3) | 3 (6.5) |
| Biliary tract cancer (BTC) | 16 | 1 (6.3) | 15 (93.7) | 0 (0.0) |
| Pancreatic cancer (PC) | 6 | 1 (16.7) | 5 (83.3) | 0 (0.0) |
| Sarcoma | 37 | 0 (0.0) | 35 (94.6) | 2 (5.4) |
| Melanoma | 8 | 0 (0.0) | 7 (87.5) | 1 (12.5) |
| Hepatocellular carcinoma (HCC) | 15 | 0 (0.0) | 15 (100.0) | 0 (0.0) |
| Other | 14 | 0 (0.0) | 14 (100.0) | 0 (0.0) |
+, claudin 18.2 immunohistochemical membrane staining in >5% of tumor cells; −, claudin 18.2 immunohistochemical membrane staining negative or less than 5% of tumor cells.
Correlation between the claudin 18.2 status and clinicopathologic features in GC
Among the 17 patients positive for claudin 18.2, the majority (12) had some form of GC. Thus, we analyzed the correlation between claudin 18.2 positivity and clinicopathologic features in GC. Four tumor samples from 85 patients with GC (4.7%) were not sufficient to analyze claudin 18.2 expression by IHC. Table 3 shows that there was no statistical difference in the various clinicopathologic features between tumors with and without claudin 18.2 expression. Only Lauren classification was observed to be different according to the claudin 18.2 status.
Table 3
| Variables | Claudin 18.2+ (n=12) | Claudin 18.2− (n=69) | P value |
|---|---|---|---|
| Gender, n (%) | 0.755 | ||
| Male | 8 (66.7) | 41 (59.4) | |
| Female | 4 (33.3) | 28 (40.6) | |
| Age, n (%) | >0.999 | ||
| ≤65 | 9 (75.0) | 51 (73.9) | |
| >65 | 3 (25.0) | 18 (26.1) | |
| Disease extent, n (%) | 0.097 | ||
| Locally advanced disease | 7 (58.5) | 21 (30.4) | |
| Metastatic disease | 5 (41.7) | 48 (69.6) | |
| Tumor site, n (%) | 0.106 | ||
| Cardia | 0 (0.0) | 3 (4.3) | |
| Body | 10 (83.3) | 33 (47.8) | |
| Antrum | 2 (16.7) | 33 (47.8) | |
| Pathologic differentiation, n (%) | 0.886 | ||
| Well | 0 | 0 | |
| Moderate | 4 (33.3) | 23 (33.3) | |
| Poor | 7 (58.7) | 32 (46.4) | |
| Mucinous | 0 (0.0) | 2 (2.9) | |
| Signet ring cell type | 1 (8.3) | 12 (17.4) | |
| Lauren classification, n (%) | 0.026 | ||
| Intestinal type | 4 (33.3) | 20 (29.0) | |
| Diffuse type | 2 (16.7) | 37 (53.6) | |
| Mixed type | 5 (41.7) | 10 (14.5) | |
| NE | 1 (8.3) | 2 (2.9) | |
| HER2 status, n (%) | 0.095 | ||
| Negative | 8 (66.7) | 60 (87.0) | |
| Positive | 4 (33.3) | 9 (13.0) | |
| EBV status (n=75), n (%) | 0.101 | ||
| Negative | 9/12 (75.0) | 59/69 (85.5) | |
| Positive | 3/12 (25.0) | 4/69 (5.8) | |
| NE | 0/12 (0.0) | 6/69 (8.7) |
HER2, human epidermal growth factor receptor 2; EBV, Epstein-Barr virus.
Impact of claudin 18.2 expression on survival of patients with metastatic solid cancer types
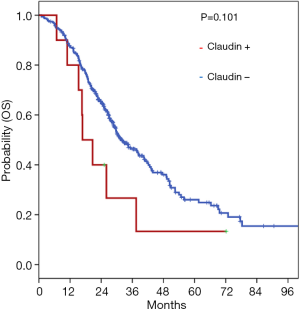
Among patients with metastatic solid cancer tumors, the influence of claudin 18.2 expression on survival was analyzed, based on the anatomic tumor type. Data were available for 325 metastatic solid cancer tumors including CRC, GC, BTC, PC, and GU/miscellaneous cancers. There was no significant difference in overall survival (OS) between patients with and without claudin 18.2 expression (P=0.101) (Figure 2).
Discussion
The identification of novel targets for drugs is paramount in precision medicine. Novel targets are capable of identifying patients most likely to benefit from a given therapy, while sparing potential physical and socioeconomic consequences for those unlikely to benefit. Recently, claudin 18.2 has been considered as an emerging novel target and a new agent selectively targeting claudin 18.2 has been developed (3,22,23). However, there are little data available on the expression of claudin 18.2 across tumor types. In the present study, we identified claudin 18.2 expression in 4.1% of 430 patients with various solid tumors, and 14.1% of 85 patients with GC showed an expression of claudin 18.2.
Claudins are major tight junction proteins; they comprise at least 27 member proteins that are expressed in a tissue-specific manner (23,24). Among the various types of claudin proteins, claudin 18.2 has been the most widely studied across several tumor types, including GC and PC (8,15,22), especially after the development of zolbetuximab and after its clinical trials showed promising outcomes (8,9,17). GC is a heterogeneous disease that shows different biological behaviors in ethnic subgroups, with varying tumor responses to targeted agents. In the present study of Korean population, the expression of claudin 18.2 was observed in 14.1% of patients with GC. This relatively low prevalence disagrees with previous reports, and there are several possible reasons (8,9,18). First, the heterogeneity of the clinicopathologic features of the analyzed patient populations among studies. Second, the antibody against claudin 18.2 used for IHC testing was not standardized; thus, the antibodies used against claudin 18.2 in each study were different (19,20,25,26). Third, standard criteria for the expression of claudin 18.2 have not yet been established.
Changes in claudins at tight junctions are related to damages of tight adhesion and polarity in the epithelia. These structural abnormalities can cause increased cellular proliferation, epithelial-mesenchymal transition, invasion, and metastasis (12,27,28). Therefore, the loss of claudin 18.2 was seen as an indicator of poor prognosis in some tumor types (29). We analyzed the prognostic role of claudin 18.2 in 325 patients with solid tumors that had been evaluated for their claudin 18.2 status. Our analysis revealed that there was no significant difference in OS according to the status of claudin 18.2 expression.
Our data expand the current knowledge of claudin 18.2 expression by analyzing various tumor types. In the present study, a relatively low prevalence (4.1% from a cohort of 430 patients) of claudin 18.2 expression was reported for all tumor types compared with previous studies (16,30). Previous studies revealed a prevalence of 50% or more claudin 18.2-positive expression in GC and PC. Patient heterogeneity, antibody differences, and lack of established testing criteria may also explain this discrepancy. In particular, a standard antibody and defined pathological criteria to detect the expression of claudin 18.2 must be established for the clinical application of claudin 18.2 as a novel biomarker.
Currently, claudin 18.2 is considered a novel target in various tumor types. Zolbetuximab is a promising agent against claudin 18.2 expressed in patients with cancer. As zolbetuximab showed remarkable success against GC, it should be considered for extended clinical exploration in the treatment of other cancers. Overall, our results add to the emerging literature about the expression of claudin 18.2 in various cancer types and support the need for extended clinical exploration of zolbetuximab. A prospective basket trial assessing and treating claudin 18.2-expressing tumors with zolbetuximab would be an interesting study to advance precision medicine.
Acknowledgments
Funding: This work was supported by funding from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-1876). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board of the Samsung Medical Center (Seoul, Korea) (No. 2013-10-017), and all patients provided written informed consent before enrollment. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 2004;286:C1213-28. [Crossref] [PubMed]
- Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci 2001;16:126-30. [PubMed]
- Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008;14:7624-34. [Crossref] [PubMed]
- Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res 2005;65:9603-6. [Crossref] [PubMed]
- González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 2008;1778:729-56.
- Mullin JM, Laughlin KV, Ginanni N, et al. Increased tight junction permeability can result from protein kinase C activation/translocation and act as a tumor promotional event in epithelial cancers. Ann N Y Acad Sci 2000;915:231-6. [Crossref] [PubMed]
- Okugawa T, Oshima T, Chen X, et al. Down-regulation of claudin-3 is associated with proliferative potential in early gastric cancers. Dig Dis Sci 2012;57:1562-7. [Crossref] [PubMed]
- Rohde C, Yamaguchi R, Mukhina S, et al. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol 2019;49:870-6. [Crossref] [PubMed]
- Türeci O, Sahin U, Schulze-Bergkamen H, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol 2019;30:1487-95. [Crossref] [PubMed]
- Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer 2006;6:186. [Crossref] [PubMed]
- Ouban A, Ahmed AA. Claudins in human cancer: a review. Histol Histopathol 2010;25:83-90. [PubMed]
- Turksen K. Claudins and cancer stem cells. Stem Cell Rev Rep 2011;7:797-8. [Crossref] [PubMed]
- Ueda J, Semba S, Chiba H, et al. Heterogeneous expression of claudin-4 in human colorectal cancer: decreased claudin-4 expression at the invasive front correlates cancer invasion and metastasis. Pathobiology 2007;74:32-41. [Crossref] [PubMed]
- Wöll S, Schlitter AM, Dhaene K, et al. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer 2014;134:731-9. [Crossref] [PubMed]
- Micke P, Mattsson JS, Edlund K, et al. Aberrantly activated claudin 6 and 18.2 as potential therapy targets in non-small-cell lung cancer. Int J Cancer 2014;135:2206-14. [Crossref] [PubMed]
- Jun KH, Kim JH, Jung JH, et al. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg 2014;12:156-62. [Crossref] [PubMed]
- Sahin U, Schuler M, Richly H, et al. A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer. Eur J Cancer 2018;100:17-26. [Crossref] [PubMed]
- Sahin U, Tureci Ö, Manikhas GM, et al. Zolbetuximab combined with EOX as first-line therapy in advanced CLDN18.2+ gastric (G) and gastroesophageal junction (GEJ) adenocarcinoma: Updated results from the FAST trial. J Clin Oncol 2019;37:abstr 16.
- Karanjawala ZE, Illei PB, Ashfaq R, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol 2008;32:188-96. [Crossref] [PubMed]
- Tanaka M, Shibahara J, Fukushima N, et al. Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J Histochem Cytochem 2011;59:942-52. [Crossref] [PubMed]
- Birungi J, Mills EJ. Can we increase male involvement in AIDS treatment? Lancet 2010;376:1302. [Crossref] [PubMed]
- Türeci O, Koslowski M, Helftenbein G, et al. Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene 2011;481:83-92. [Crossref] [PubMed]
- Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol 2000;149:13-6. [Crossref] [PubMed]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001;2:285-93. [Crossref] [PubMed]
- Liu H, Shi J, Anandan V, et al. Reevaluation and identification of the best immunohistochemical panel (pVHL, Maspin, S100P, IMP-3) for ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med 2012;136:601-9. [Crossref] [PubMed]
- Soini Y, Takasawa A, Eskelinen M, et al. Expression of claudins 7 and 18 in pancreatic ductal adenocarcinoma: association with features of differentiation. J Clin Pathol 2012;65:431-6. [Crossref] [PubMed]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 2006;22:207-35. [Crossref] [PubMed]
- Hollande F, Blanc EM, Bali JP, et al. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am J Physiol Gastrointest Liver Physiol 2001;280:G910-21. [Crossref] [PubMed]
- Matsuoka T, Mitomi H, Fukui N, et al. Cluster analysis of claudin-1 and -4, E-cadherin, and beta-catenin expression in colorectal cancers. J Surg Oncol 2011;103:674-86. [Crossref] [PubMed]
- Oshima T, Shan J, Okugawa T, et al. Down-regulation of claudin-18 is associated with the proliferative and invasive potential of gastric cancer at the invasive front. PLoS One 2013;8:e74757. [Crossref] [PubMed]


