
Recombinant Newcastle disease virus expressing human IFN-λ1 (rL-hIFN-λ1) inhibits lung cancer migration through repolarizating macrophage from M2 to M1 phenotype
Introduction
Lung cancer is a serious threat to human health and has been the most common cause of cancer-related death worldwide. The 5-year survival rate of lung cancer patients diagnosed at a distant stage is as low as 5% (1). The low survival rate of lung cancer patients is much closely related with the uncontrolled proliferation and metastasis of cells within the lung (2). Radiotherapy and chemotherapy can improve the 5-year survival rate of patients with lung cancer to a certain extent, but the effect is limited. Immune escape or immune tolerance seriously affects the therapeutic effect of lung cancer. In recent years, tumor immunotherapy has gradually entered people's field of vision, which is another great breakthrough in the development of science and technology, and has become a new hot spot in tumor treatment.
Tumor microenvironment (TME) plays an important role in the development and malignant evolution of tumors. The tumor microenvironment contains many resident cell types, such as adipocytes and fibroblasts, but it is also populated by migratory haematopoietic cells, most notably macrophages, neutrophils and mast cells (3). Macrophages are the most frequent immune cells occurring in the tumor microenvironment and affect the tumor progression and metastasis (4). As part of the innate immune system, macrophages are crucial cells with high plasticity and heterogeneity that play a significant role in defensing against bacterial, viral, and parasitic infection in microenvironment (5).
According to the immunological study, polarized macrophages can be divided into two functionally distinct states, namely classically activated macrophages or M1 and alternatively activated macrophages or M2, each is related to specific immune responses, among which both progression and resolution of inflammation constitute a critical determinant (6).
M1 macrophages, the classically activated proinflammatory macrophages, were induced when exposed to classical activators such as lipopolysaccharides (LPS), inducible nitric oxide synthase (iNOS) and IFN-γ, expressing high levels of iNOS, IL-6, CXC ligand 10 (CXCL10) and TNF-α. M2 macrophages, alternatively activated macrophages, were induced when exposed to alternative activators such as interleukin IL-4, IL-10, IL-21, activin A, immune complexes and IL-13, and they showed anti-inflammatory properties by suppressing the production of inflammatory cytokines and expressing arginase-1 (Arg-1), mannose receptor, galactose receptor and CD163 antigen instead of iNOS (7,8).
M1 macrophages have roles in inflammation factors secretion and antitumor immunity, resulting in protecting the host from a variety of bacteria and viruses, while M2 macrophages contribute to metastasis by stimulating tumor proliferation, invasiveness and angiogenesis (9). Macrophages within the tumor microenvironment termed tumor-associated macrophages (TAMs) are known to be crucial cells in lung cancer as they are in close proximity to tumor cells compared with other stromal cells (10). Based on extensive studies, it has been proposed that high infiltration of M1 macrophages in the tumor islets and low infiltration of tumor-polarization M2 macrophages are associated with improved NSCLC patients’ prognosis (11).
Interleukin-29 (IL-29), also labeled as IFN-λ1, is a new member of the type III IFN family recently discovered (12). Previous studies have identified the receptor for IL-29 (IL-28R) is expressed on a more restricted number of cells than IFN-α, including colon, prostate, and lung, which ensure it may be the more targeted therapy (13). IL-29, with dual function in the immune system, could increase production of the Th1 cytokine(e.g., IFN-γ, IL-6, IL-8, and IL-10) and reduce the Th2 cytokines (e.g., IL-4, IL-13) in macrophages resulting in a shift from a type II T helper cell response to a type I T helper cell response (14).
Recombinant adenoviruses expressing IFN- λ 1 can up-regulate the expression of MHC I molecules on the surface of cells, promote the production of CD8+ T cells, increase the cytotoxicity of T cells in tumors, and enhance the immune response. In addition, direct intratumoral administration of either AdIL-28A or AdIL-29 mediates effective anti-tumor immune responses to significantly inhibit the growth of established tumors (15).
Furthermore, our team had successfully constructed the recombinant Newcastle disease virus expressing human IFN-λ1 named as rL-hIFN-λ1 and verified it could modulate the human Th1/Th2 immune response process and change the tumor microenvironment to an antitumor cytokine which led to the reducing of tumor growth (16). However, the correlation between rL-hIFN-λ1 and macrophage polarization and its effect on lung cancer progression remains largely unknown.
In the present study, we demonstrated that rL-hIFN-λl could be involved in M1 but not M2 macrophage polarization, resulting in the immunoregulation function in lung cancer and the inhibition of tumor cells proliferation and migration. Moreover, the correlation between macrophages polarization phenotype and clinical stage of lung cancer tissues were also investigated. Overall, our findings suggest that rL-hIFN-λl has a potential to improve the tumor microenvironment and emphasize the importance of macrophage polarization in cancer progress. This study provides an important rationale for the development of a novel therapeutic strategy in lung cancer patients through the skewing of TAMs phenotype.
Methods
Patients and tissue samples
Eighteen patients who underwent partial or radical surgical resection at the Department of Thoracic Surgery, The Affiliated People’s Hospital of Jiangsu University (China), between January 2017 and January 2018 were enrolled in this study. The patients with a history of another kind of cancer and any preoperative chemotherapy or radiotherapy were excluded before sampling. The eighteen tissue specimens were confirmed by histopathological examination. The pathological diagnosis was in accordance with tumor-node-metastasis classification for the stage (2017 Union for International Cancer Control, UICC) and histopathological grade (WHO 2015). Informed consent was obtained from each subject included in the study and the research was approved and supervised by the Ethics Committee of The Affiliated People’s Hospital of Jiangsu University.
Materials
The recombinant Newcastle disease virus expressing human IFN-λ1 (rL-hIFN-λ1)was successfully constructed with the kind help of the Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, which also provided the wild-type NDV LaSota strain. Cell culture reagents were acquired from Gibco (Grand Island, NY, USA). IFN-γ and IL-4 were purchased from PeproTech (Rocky Hill, NJ). Phorbol 12-myristate13-acetate (PMA, Sigma, P1585) and Lipopolysaccharide (LPS, E. coli 055:B5) were obtained from Sigma-Aldrich (St. Louis, MO). Transwell unit was from Millipore (Bedford, MA).
Cell lines and cell culture conditions
The human lung adenocarcinoma A549 cell line and human small cell lung cancer H446 cell line were purchased from the Cancer Cell Repository (Shanghai Cell Bank, Shanghai, China; 2010-02-20). The human THP-1 cell line was purchased from the American Type Culture Collection (ATCC; Rockville, MD, USA). All the cells were cultured in RPMI 1640 (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, HyClone), 100 mg U/mL penicillin, and 100mg U/ml streptomycin at 37 ºC, 5% CO2 in a humidified incubator.
Construction of THP-1 derived M0/M1/M2 macrophage model
THP-1 monocytes were differentiated into macrophages by the way that previously described. Briefly, to obtain attached THP-1-M0 macrophages, THP-1 cells (1.0×106/well) were seeded into 6-well plates cultured in RPMI 1640 medium containing 10% FBS and 100 ng/ml phorbol 12-myristate13-acetate (PMA, Sigma, P1585) for 24 h. For THP-1-M1 or THP-1-M2 macrophages polarization, THP-1-M0 macrophages were further incubated with 10 ng/mL Lipopolysaccharide (LPS, E. coli 055:B5) plus 20 ng/mL IFN-γ or 20 ng/mL IL-4 inRPMI-1640 with 10% fetal bovine serum (FBS, HyClone) for another 24 h, respectively. Additionally, NDV was added to THP-1-M0 macrophages at the indicated MOI to obtain the THP-1-NDV macrophages and rL-hIFN-λ1 was introduced into the THP-1-M0 macrophages at a MOI of 1.0 to obtain the THP-1-rL-hIFN-λ1 macrophages (16). For the indicated experiments, the supernatants of each subset of macrophages were harvested, named as CM-THP-1-M0, CM-THP-1-M1, CM-THP-1-M2, CM-THP-1-NDV, CM-THP-1-rL-hIFN-λ1, centrifuged and stored at –80 °C for further use.
Quantitative RT-PCR
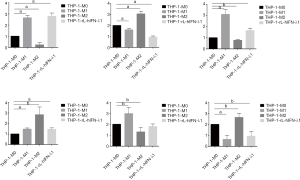
After the differentiation and polarization were performed as indicated, total RNA was extracted from each subtype of the polarized macrophage cells using Trizol reagent according to the manufacturers’ instructions (Invitrogen, Carlsbad, USA). The reverse transcription was performed using a reverse transcription kit (Takara, Otsu, Japan) to amplify CD86, TNF-α, iNOS, CD163, Arg1, and Mrc1. Then Real-time PCR was conducted on a BIO-RADCFX96-TmReal-Time System by mixing 2 µL cDNA with SYBR Green Master Mix (Applied Biosystems, USA). The forward and reverse primers were synthesized in GenePharma (Shanghai, China). The sequence of primers was shown in the Table 1. The relative expression of target mRNAs were normalized to GAPDH and quantified using the 2-ΔΔCtmethod.
Table 1
| Gene | Primer sequences | PCR product (bp) |
|---|---|---|
| GAPDH | CAGGAGGCATTGCTGATGAT | 77 |
| GAAGGCTGGGGCTCATT T | ||
| CD86 | TGCTCATCTATACACGGTTACC | 137 |
| TGCATAACACCATCATACTCGA | ||
| CD163 | ATCAACCCTGCATCTTTAGACA | 80 |
| CTTGTTGTC ACATGTGATCCAG | ||
| iNOS | ACTTTCCAAGCACACTTCAC | 361 |
| TTCGATAGCTTGAGGTAGAAGC | ||
| TNF-α | CAATGGCGTGGAGCTGAGAG | 152 |
| TCTGGTAGGAGACGGCGATG | ||
| Arg1 | CATCCCTAATGACAGTCCCTTT | 210 |
| CAGGAGGAAAGATACAGGTT GT | ||
| Mrc1 | GACGTGGCTGTGG ATAAATAAC | 104 |
| CAGAAGACGCATGTAAAGCTAC |
Immunofluorescence analysis
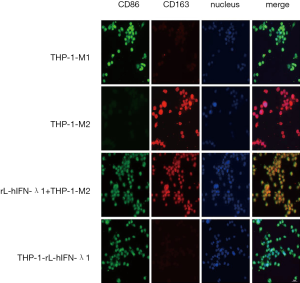
THP-1-M1, THP-1-M2, THP-1-NDV and THP-1- rL-hIFN-λ1 macrophages (5×105 per well) were induced as described above. Additionally, in order to investigate the function of rL-hIFN-λ1 on M2 macrophages polarization, the rL-hIFN-λ1 at a MOI of 1.0 was introduced to M2 macrophages. Then all the cells were washed in PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) at 4 °C for 30 min. The 0.1% Triton X-100 was used for permeabilization and then blocked with10% FBS to eliminate the nonspecific fluorescence. Immunofluorescence staining was performed using CD86 (1:200, Cell Signaling Technology, CST, Beverly, MA, USA), and CD163 (1:200, Proteintech) as the primary antibodies, and then the cell preparations were incubated with DyLight 488/594 labeled secondary antibodies (Beyotime Biotechnology, Jiangsu, China). After washing with PBS, cells were examined with the fluorescence microscope.
Clonogenic survival assay
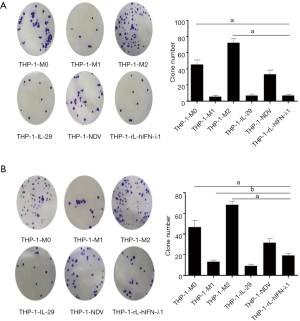
A549 and H446 cells were plated in six-well plates and cultured overnight (500 cells per well) before being transfected with or without the supernatants of each subunit of macrophages. After the cells became attached, the CM was added, then these cells were cultured for 24 h, respectively. Furthermore, the medium was replaced with supernatants-free medium, several days later, cells were fixed and stained with crystal violet (0.2%) to visualize cell colonies. Each sample was repeated in 3 wells.
Scratch wound healing assay
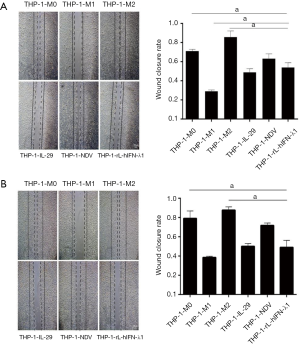
A549 cells and H446 cells were seeded in six-well plates in RPMI-1640 medium supplemented with no FBS. When cells confluence reached 50–70%, the CM of each group was introduced into the two kinds of lung cancer cells, respectively. Then avertical area was scratched using a pipette tip when cells reached 95–100%, and washed twice with PBS to remove the loose debris. The recovery of the wound (cell migration towards the wound to close the gap) was evaluated at 24 h. The images were captured using a fluorescence microscope (ECLIPSE Ti-s, Nikon, Tokyo, Japan). The images were collected from nine independent selected fields in each sample, and the wound areas were calculated by NIH Image J software (National Institutes of Health, Bethesda, MD, USA).
Invasion assay
The invasion assay was analyzed using a 24-well plate that contained transwell inserts (Corning, MA, USA). The A549 or H446 cells were suspended in serum-free RPMI-1640 medium, and 1×105 of these cells were seeded in per upper insert. Then, 600 µL CM-THP-1-M0, CM-THP-1-M1, CM-THP-1-M2, CM-THP-1-NDV, CM-THP-1-rL-hIFN-λ1 were added to the lower chamber of the corresponding groups and incubated at 37 °C, respectively. After 24 h, the upper surface of the membranes was scraped with a cotton swab, migrated cells on the lower surface of the membrane were fixed with 4% paraformaldehyde, stained with crystal violet (0.2%), and cells were counted in nine fields of vision and photographed under microscopy at 200× magnification.
Immunohistochemistry (IHC)
In total, eighteen lung cancer tissues whose histopathological grade were confirmed as I stage lung cancer tissues, II stage lung cancer tissues and III stage lung cancer tissues were obtained to perform Immunohistochemical staining, and each group contained six samples. Lung cancer tissues and pleural effusions in different clinical stages were collected and treated with 500 r/min centrifugation for 5 min, 4 µm paraffin-embedded sections were made by fixation, dehydration, transparent and conventional paraffin embedding. Samples were dewaxed in xylene, rehydrated using a graded series of ethanol solutions and incubated with 3% peroxide-methanol solution at room temperature (RT) for 10 min to block peroxidase activity. Then antigen retrieval was performed at 100 °C in an autoclave for 7 min. Sections were incubated with rabbit anti-CD86 antibody (1:200, Xinfan Biotechnology, China) and goat anti-CD163 (1:200, Xinfan Biotechnology, China) overnight at 4 °C. The next day, goat anti-rabbit IgG-HRP (1:1,000, Xinfan Biotechnology, China) and rabbit anti-goat IgG-HRP antibody (1:1,000, Xinfan Biotechnology, China) were incubated at room temperature for 30 min. After washing with PBS, samples were stained with DAB and counter-stained with hematoxylin. Then dehydration, gum seal film and microscopic examination were processed. Image-pro plus6.0 was used to scan the mean optical density of CD86 and CD163 in lung cancer tissue and pleural effusions.
Statistical analysis
All the experiments were performed independently in triplicate. The data were presented as the means ± standard error of the mean (SEM). The one-way ANOVA was used to analyze multiple groups, and the LSD-T test was used for the further comparison between the two groups. For all tests, significant differences were indicated for a: P<0.01, b: P<0.05.
Results
Cells polarization
Previous studies had identified several canonical markers concerning the specificity for each macrophage subtype (17), and we evaluated THP-1-M1/THP-1-M2 macrophage polarization by detecting the gene expression of the markers. Following a consensus in macrophage polarization that the mRNA expression of M1 macrophages markers, such as CD86, TNF-α and iNOS, were significantly upregulated in the M1 population, and the mRNA expression of M2 macrophages markers, such as CD163, Mrc-1, and Arg-1, were greater in the M2 population (Figure 1). These surface markers certificated the successful polarization of monocytes into M1 and M2 polarized macrophages. Here, we also provided evidence that THP-1-rL-hIFN-λ1 exerted strong effect on M1 macrophages polarization rather than M2 macrophages which both derived from M0 macrophages.
rL-hIFN-λ1 suppressed M2 polarization of macrophages in vitro
To further investigate the biological effect of rL-hIFN-λ1 on the regulation of the macrophage polarization, the THP-1-M1 macrophages, THP-1-M2 and THP-1-rL-hIFN-λ1 macrophages were induced as described above. Agreeing with previous results, the immunofluorescence analysis shown that the LPS/IFN-γ induced M1 macrophages had high expression of CD86 protein and low expression of CD163, while the IL-4 induced M2 macrophages had completely opposite expression. And the biomarkers of THP-1-rL-hIFN-λ1 were similar to M1 macrophages. Next, we incubated THP-1-M2 macrophages with rL-hIFN-λ1 and the surface markers CD86 and CD163 expression level were evaluated. Significant upregulation of CD86 but not CD163 was observed in the intervened THP-1-M2 macrophages when rL-hIFN-λ1 was applied, indicating that rL-hIFN-λ1 could reset CD163+ M2 macrophages toward CD86 + M1 macrophages (Figure 2).
THP-1- rL-hIFN-λ1 inhibited the proliferation of A549 and H446 cells
TAMs could produce a wide variety of growth factors such as fibroblast growth factor (FGF), hepatocyte growth factor (HGF), epidermal-growth-factor receptor (EGFR)-family ligands, platelet-derived growth factor (PDGF) and transforming growth factor-βs (TGF-βs) as well as the overexpression of macrophage growth factors or chemokines correlate with poor prognosis (3). However, the M1 macrophages could participate in the prevention of tumor proliferation procession, we hypothesized that the rL-hIFN-λ1 cytokines would be effective in suppressing lung cancer cells proliferation. To test this, we co-cultured the CM of the different macrophages with the lung cancer cells, and the proliferation ability of lung cancer cells were investigated by clonogenic survival assay. The A549 cells cocultured with the M2 CM showed significant increase in proliferation in vivo compared to M1 group and rL-hIFN-λ1 group, which suggested that the THP-1- rL-hIFN-λ1 induced macrophage modulated a tumor-suppressing microenvironment in vivo (Figure 3).
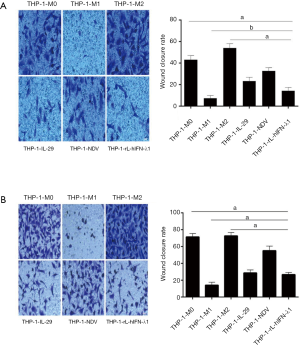
THP-1-rL-hIFN-λ1 inhibited the migration and invasion of A549 and H446 cells
Enhanced cell migration and invasion abilities have a pivotal role in cancer development, which leads to poor prognosis. As already described in literature, the proportion of M2 macrophages within the tumor is critically correlated with the metastatic potential of the tumor but the M1 macrophages have opposite relationship with the metastatic tumor progression (18). Therefore, we determined whether THP-1-rL-hIFN-λ1 could inhibit the migration and invasion of lung cancer cells as THP-1-M1 macrophages. The CMs of different polarized macrophages were harvested and co-cultured with A549 cells and H446 cells, respectively. As shown in Figure 4, CM-THP-1-M2 significantly promoted the migration of A549 and H446 cells in wound-healing assays, whereas CM-THP-1-M1 had the opposite effect. Meanwhile, CM-THP-1-rL-hIFN-λ1 successfully inhibited the migration ability of A549 cells and H446 cells which was similar to CM-THP-1-M1.
To better understand the effect of THP-1-rL-hIFN-λ1 on lung cancer cells invasion, we developed a Transwell coculture system in which lung tumor cells and harvested CMs were cocultured in the same well though separated by a 0.8 µm-poresize barrier. Confirmed with the wound-healing assays, we also observed that CM-THP-1-M2 significantly increased cell invasion in A549 and H446 cells in comparison with CM -THP-1-M0, and this effect on cell invasion was also inhibited by CM-THP-1-rL-hIFN-λ1 (Figure 5 ). These observations indicated that THP-1-rL-hIFN-λ1abrogated lung cancer cell migration and invasion in vitro.
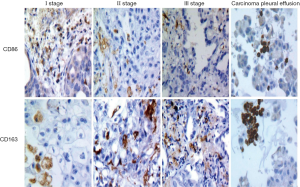
Correlation of M1and M2 macrophage infiltration with patient clinicopathological
To identify and quantify the amount of infiltrated macrophage phenotype in different clinical stages of lung adenocarcinoma tissues, we tested the expression of hallmarks of M1 and M2 macrophages in paraffin-embedded lung cancer samples by IHC. The results showed that the level of M1 macrophages in the clinical I stage lung cancer tissues was significantly higher than that in stage II and III lung cancer tissues (t=4.53, 6.82, P<0.01), while the infiltration density of M2 macrophage in the clinical I stage lung cancer tissues was significantly lower than that of clinical stage II and III lung cancer tissues (t=3.88, 6.39, P<0.01). Meanwhile, in clinical I stage lung cancer tissues the density of M1 macrophages was higher than the M2 macrophages, but clinical II and III lung cancer tissues showed the opposite trend. And the infiltration density of M1 macrophages was lower than the density of M2 macrophages in malignant pleural effusions (t=11.19, P<0.01) (Figure 6).
Discussion
Lung cancer is a malignant tumor with the highest morbidity and mortality worldwide, and the metastasis is the leading cause of its mortality and insensitivity to cancer treatment. The preference to metastasis at early stage contributes to a poor prognosis and insensitive to the therapeutic strategies (19). Despite the development of many treatment strategies, the long-term survival rate of lung cancer patients is still very low. Therefore, it is urgent to improve our understanding of the mechanisms associated with the development of lung cancer and identify novel therapeutic targets that could eventually overcome the obstacles in lung cancer treatment.
TAMs are abundant in the microenvironment of non-small cell lung cancer. It has been confirmed that the proportion of M2 TAMs in NSCLC is higher compared with those of M1 macrophages (20). The number of TAMs infiltration in cancer nests can be used as one of the independent prognostic factors of NSCLC (21). Macrophages can present, devour and deal with antigens. Their immunophenotypes can change dynamically in vivo environment at random, so they have diverse phenotypes and rich functions. Macrophages could change their phenotype in response to the surrounding cytokines in the TME. When exposed to Th1 cytokines, M0 macrophages differentiated into the “classical activated” M1 macrophages while differentiated into “alternatively activated” M2 macrophages when exposed to Th2 cytokines, which was in consistent with our results that macrophages had increased CD86 and CD163 expression when stimulated with LPS/IFN-γ and IL-4, respectively. When the specific genes in the polarization pathway of TAMs were blocked by inhibitors, the growth of NSCLC could be inhibited through enhancing the macrophage-mediated anti-tumor immunity (22). TAMs not only participated in the malignant evolution of lung cancer, but also regulated the mechanism of chemotherapy resistance of lung cancer, and affected the therapeutic effect of lung cancer (23,24).
In the present research, human peripheral blood monocyte THP-1 was used to construct the macrophage polarization model, because it had biological characteristics that other monocytes did not possessed, and it could produce a variety of cytokines and enzymes similar to those in the internal environment (25). CD86 is a highly glycosylated lysosom.al membrane protein, which is mainly expressed in the cytoplasm of macrophages. At present, CD86 is widely used as a molecular marker of M1 TAMs. CD163 is an important member of scavenging receptor superfamily, which is mainly expressed on the surface of monocytes and macrophages. Many studies have confirmed that CD163 protein is a highly specific macrophage marker associated with M2 TAMs (21). Therefore, CD86 and CD163 were used to label M1 macrophages and M2 macrophages, respectively. In addition, consistent with the previous report that THP-1-M1 macrophages showed higher levels of proinflammatory cytokine mRNA expression, such as TNF-α and iNOS than THP-1-M0 or THP-1-M2 macrophages. In contrast, THP-1-M2 macrophages showed markedly elevated expression of MRC1 and Arg-1. It was confirmed that THP-1-derived macrophages could obtained different characteristics after polarization.
Selectively programming or re-educating macrophages toward a tumor-suppressor phenotype can be a potential therapeutic strategy (26). Given the phenotypic plasticity of macrophages, it is possible to reprogram M2 macrophages to develop an M1 phenotype by increasing the environmental concentration of anti-tumor cytokines (27). NDV, an oncolytic virus, has been confirmed to have the potency of antitumor through immunomodulatory mechanisms (28). Our team inserted human IFN-λ1 gene into the NDV viral genome in order to gain the rL-hIFN-λ1 which stably expressing IFN-λ1 (16). The IL-28 receptor chain (IL-28RA) and the IL-10 receptor 2 chain (IL-10R2) are the two receptors of IFN-λ, especially IL-28RA is not expressed by human primary monocytes and monocyte-derived DCs, it is expressed by monocyte-derived macrophages. It has been well recognized that IFN-λ1 enhances IFN-γ-induced IL-12 production by human monocyte-derived macrophages via differential regulation of IFNγR1 expression (29). Besides, IL-12 has been confirmed to inhibit tumor growth by inducing macrophages polarization to M1- like phenotype through downregulating the p-STAT3 and c-myc expression and play the effective anticancer action by inhibiting the growth of cancer cell in hepatocellular carcinoma (30). Therefore, we speculated that rL-hIFN-λl could mobilize the immune response and exert antitumor effect by inducing the polarization of M0 macrophages to M1 macrophages.
The findings of this study showed that treatment with rL-hIFN-λ1 into M0 macrophages inhibited the polarization of M2 type macrophages, meanwhile, rL-hIFN-λ1-induced phenotype switched to M1 type macrophages. This is in line with the results in vitro, the up-regulation of anti-inflammatory M2 makers Arg1 and CD206 induced by IL-4 were all reduced upon rL-hIFN-λ1 treatment. And, rL-hIFN-λ1 further increased levels of the pro-inflammatory M1 markers, iNOS and TNF-α in the present of LPS/IFNγ treatment. However, the mechanism of rL-hIFN-λ1 induced polarization of THP-1 cells to M1 macrophages has not been confirmed, which still needs to be further discussed.
It was recently reported that TAMs were primarily polarized into M2 macrophages and were associated with cancer progression and metastasis (31). Clinical studies and experimental evidence suggest that M2-polarized macrophages can promote tumor migration, invasion, metastasis, and angiogenesis by secreting growth and angiogenesis factors, remodeling extracellular matrix, and suppressing immune responses. In contrast, M1-activated macrophages could reduce tumor progression and inhibit tumor growth through direct action, such as secretion of ROS, or promotion of the Th1 response (32,33). M1 macrophages attempt to kill tumor cells via contact-dependent mechanisms or through molecule secretion, while M2 macrophages tend to exhibit a pro-tumor phenotype characterized by the release of immunosuppressive and angiogenic agents that allow tumor to survive and grow. Previous studies had identified that IFN-λ1 inhibited the growth of several tumour cells, such as a neuroendocrine tumour as well as glioblastoma and colon cancer cell lines (34). It has been discovered that IL-29 provided anti-proliferative effects by upregulating the p21 molecule in cells through its STAT signaling pathways in human non-small cell lung cancer (35). Agreeing with previous studies, our result showed that M2 macrophages enhanced lung cancer growth and migration while M1 macrophages showed the opposite trend. Besides, THP-1-rl-hIFN-λl could inhibit proliferation, colony formation and migration of lung cancer cells in vitro. Taken together, these results further supported the conclusion that the antitumor effect of rL-hIFN-λ1 depended on the macrophage polarization.
M2 macrophages, the main component of tumor matrix, affect the long-term prognosis of tumor. The more M2 macrophages predict the worse prognosis, while the more M1 macrophages predict the better the prognosis (36,37). In the present study, the numbers of M1 and M2 macrophages in tumor tissue within different clinical stages were examined. The IHC results showed that the M1 macrophage accumulation was richer than M2 macrophage in lung cancer tissues with less malignancy which indicated that the enrichment of M1 macrophages forecasting better prognosis. Therefore, it was suggested that the clinical stage and progress of lung cancer were closely related to the polarization phenotype of TAMs infiltrating in it. The polarization of TAMs in lung adenocarcinoma can be used as a reference for guiding the clinical stage of tumor and judging the clinical prognosis of tumor patients.
Conclusions
Altogether, our results indicate that M2 macrophages are rich in lung adenocarcinoma tissues and rL-hIFN-λl induces M1 macrophage differentiation (TNF-α high iNOS high Arg1 low Mrc1 low), furthermore the rL-hIFN-λl-induced M1 macrophages exert anti-tumor function by prevent lung cancer cell proliferation and migration. The ability to mediate the macrophage polarization and inhibit lung cancer cell proliferation and migration will make rL-hIFN-λl an important consideration for anti-lung cancer and immunotherapy research moving into the future.
Acknowledgements
The authors would like to thank to Zhijian Zhang, Jiangsu University, China for excellent experimental assistance. Special thanks also to Xi Wei for the provision of immunohistochemistry techniques.
Funding: This work was supported by Grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2320). The authors have no conflicts of interest to declare.
Ethics statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This project was approved and supervised by the Ethics Committee of the Affiliated People’s Hospital of Jiangsu University. All patients included in this study provided written informed consent before surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Dinglin X, Ding L, Li Q, et al. RYBP Inhibits Progression and Metastasis of Lung Cancer by Suppressing EGFR Signaling and Epithelial-Mesenchymal Transition. Transl Oncol 2017;10:280-7. [Crossref] [PubMed]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews Cancer 2004;4:71-8. [Crossref] [PubMed]
- Li W, Zhang X, Wu F, et al. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis 2019;10:918. [Crossref] [PubMed]
- Tang MX, Hu XH, Liu ZZ, et al. What are the roles of macrophages and monocytes in human pregnancy? J Reprod Immunol 2015;112:73-80. [Crossref] [PubMed]
- Bamodu OA, Kuo KT, Wang CH, et al. Astragalus polysaccharides (PG2) Enhances the M1 Polarization of Macrophages, Functional Maturation of Dendritic Cells, and T Cell-Mediated Anticancer Immune Responses in Patients with Lung Cancer. Nutrients 2019;11:2264. [Crossref] [PubMed]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13. [Crossref] [PubMed]
- Shayan M, Padmanabhan J, Morris AH, et al. Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization. Acta Biomater 2018;75:427-38. [Crossref] [PubMed]
- Wu JY, Huang TW, Hsieh YT, et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol Cell 2020;77:213-27.e5. [Crossref] [PubMed]
- Shih JY, Ang Y, Chen JJW, et al. Tumor-Associated Macrophage: Its Role in Cancer Invasion and Metastasis. J Cancer Mol 2006;2:101-6.
- Jackute J, Zemaitis M, Pranys D, et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol 2018;19:3. [Crossref] [PubMed]
- Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 2010;30:555-64. [Crossref] [PubMed]
- Guenterberg KD, Grignol VP, Raig ET, et al. Interleukin-29 Binds to Melanoma Cells Inducing Jak-STAT Signal Transduction and Apoptosis. Mol Cancer Ther 2010;9:510-20. [Crossref] [PubMed]
- Dai J, Megjugorac NJ, Gallagher GE, et al. IFN- 1 (IL-29) inhibits GATA3 expression and suppresses Th2 responses in human naive and memory T cells. Blood 2009;113:5829-38. [Crossref] [PubMed]
- Hasegawa K, Tagawa M, Takagi K, et al. Anti-tumor immunity elicited by direct intratumoral administration of a recombinant adenovirus expressing either IL-28A/IFN-λ2 or IL-29/IFN-λ1. Cancer Gene Therapy 2016;23:266. [Crossref] [PubMed]
- Bu X, Li M, Zhao Y, et al. Genetically engineered Newcastle disease virus expressing human interferon-lambda1 induces apoptosis in gastric adenocarcinoma cells and modulates the Th1/Th2 immune response. Oncol Rep 2016;36:1393-402. [Crossref] [PubMed]
- Li M, Lai X, Zhao Y, et al. Loss of NDRG2 in liver microenvironment inhibits cancer liver metastasis by regulating tumor associate macrophages polarization. Cell Death Dis 2018;9:248. [Crossref] [PubMed]
- Yang J, Zhang Z, Chen C, et al. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene 2014;33:3014-23. [Crossref] [PubMed]
- Sun L, Chen B, Jiang R, et al. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell Immunol 2017;311:86-93. [Crossref] [PubMed]
- Sumitomo R, Hirai T, Fujita M, et al. M2 tumor-associated macrophages promote tumor progression in non-small-cell lung cancer. Exp Ther Med 2019;18:4490-8. [PubMed]
- Kim KJ, Wen XY, Yang HK, et al. Prognostic Implication of M2 Macrophages Are Determined by the Proportional Balance of Tumor Associated Macrophages and Tumor Infiltrating Lymphocytes in Microsatellite-Unstable Gastric Carcinoma. Plos One 2015;10:e0144192. [Crossref] [PubMed]
- Standiford TJ, Kuick R, Bhan U, et al. TGF-beta-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth. Oncogene 2011;30:2475-84. [Crossref] [PubMed]
- Baghdadi M, Wada H, Nakanishi S, et al. Chemotherapy-Induced IL34 Enhances Immunosuppression by Tumor-Associated Macrophages and Mediates Survival of Chemoresistant Lung Cancer Cells. Cancer Res 2016;76:6030-42. [Crossref] [PubMed]
- Chung FT, Lee KY, Wang CW, et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer 2012;131:E227-35. [Crossref] [PubMed]
- McCanna DJ, Barthod-Malat AV, Gorbet MB. In vitro methods of assessing ocular biocompatibility using THP-1-derived macrophages. Cutan Ocul Toxicol 2015;34:89-100. [Crossref] [PubMed]
- Nielsen SR, Quaranta V, Linford A, et al. Corrigendum: Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol 2016;18:822. [Crossref] [PubMed]
- Morales V, Soto-Ortiz L. Modeling Macrophage Polarization and Its Effect on Cancer Treatment Success. Open J Immunol 2018;08:36-80. [Crossref]
- Park MS, Shaw ML, Muñoz-Jordan J, et al. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J Virol 2003;77:1501-11. [Crossref] [PubMed]
- Liu BS, Janssen HL, Boonstra A. IL-29 and IFNα differ in their ability to modulate IL-12 production by TLR-activated human macrophages and exhibit differential regulation of the IFNÎ3 receptor expression. Blood 2011;117:2385-95. [Crossref] [PubMed]
- Wang Q, Cheng F, Ma TT, et al. Interleukin-12 inhibits the hepatocellular carcinoma growth by inducing macrophage polarization to the M1-like phenotype through downregulation of Stat-3. Mol Cell Biochem 2016;415:157-68. [Crossref] [PubMed]
- Sugimoto M, Mitsunaga S, Yoshikawa K, et al. Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. Eur J Cancer 2014;50:1900-8. [Crossref] [PubMed]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889-96. [Crossref] [PubMed]
- Ubil E, Caskey L, Holtzhausen A, et al. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J Clin Invest 2018;128:2356-69. [Crossref] [PubMed]
- Kelm NE, Zhu Z, Ding VA, et al. The role of IL-29 in immunity and cancer. Crit Rev Oncol Hematol 2016;106:91-8. [Crossref] [PubMed]
- Barrera L, Montes-Servin E, Barrera A, et al. Cytokine profile determined by data-mining analysis set into clusters of non-small-cell lung cancer patients according to prognosis. Ann Oncol 2015;26:428-35. [Crossref] [PubMed]
- Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Letters 2013;332:3-10. [Crossref] [PubMed]
- Xu F, Cui W, Wei Y, et al. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res 2018;37:207. [Crossref] [PubMed]


