The efficacy and safety of PD-1/PD-L1 inhibitors in breast cancer: a systematic review and meta-analysis
Introduction
Despite 5-year overall survival rate of breast cancer was 90%, approximately 30% breast cancer patients with an early-stage diagnosis eventually progressed to advanced metastatic disease, and about 6% of patients were metastatic disease at diagnosis (1). Treatments of advanced breast cancer include chemotherapy, endocrine-based therapeutic strategies, HER2-related regimens, CDK4/6 inhibitors and Poly (ADP-ribose) polymerase (PARP) inhibitors (2). The two most important targets for breast cancer are HER2 and CDK4/6. For HER2 positive breast cancer, trastuzumab greatly improves survival outcomes (3). CDK4/6 inhibitors, including palbociclib, ribociclib, and abemaciclib, combined with endocrine therapies could be suggested as the core treatment modality in patients with hormone receptor positive advanced breast cancer (4,5). Additionally, three PARP inhibitors, olaparib, rucaparib, and niraparib, have received approval for advanced cancers with breast cancer type 1/2 susceptibility protein (BRCA1/2) mutations, but the efficacy in breast cancer patients remains controversial (6-8). For triple-negative breast cancer (TNBC) lacking the expression of estrogen (ER), progesterone receptor (PR), and HER-2, cytotoxic chemotherapy is the standard treatment. However, the treatment is limited by considerable toxicity and short duration of response (9-11).
Given the suboptimal outcomes with traditional chemotherapy, new targeted therapeutic regimens for breast cancer are urgently needed. Fortunately, immune checkpoint inhibitors, including programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors, have revolutionized cancer therapy (12,13). To date, the US Food and Drug Administration has approved three PD-1 inhibitors (pembrolizumab, nivolumab and cemiplimab) and three PD-L1 inhibitors (atezolizumab, durvalumab and avelumab). Blocking the PD-1/PD-L1 pathway with monoclonal antibodies might be one means of restoring immune surveillance and T cell-mediated antitumor immunity (13). Substantial researches showed that PD-L1 was expressed in multiple solid tumors and might be a predictor of response to PD-1/PD-L1 axis inhibition (14-16). Approximately half of breast cancers expressed PD-L1, with expression generally higher in TNBC (17-22). Moreover, it was reported that, in patients with TNBC, PD-1 occurred mainly on tumor-infiltrating immune cells (19,23). Thus, both the PD-1 and PD-L1 inhibitors might be useful therapeutic regimens for breast cancer.
To date, many single-arm clinical trials have reported the benefits and toxicities of PD-1/PD-L1 inhibitors for breast cancer without control therapies. Most of the trials found that PD-1/PD-L1 inhibitors provided durable clinical benefit and were well tolerated with or without combined treatment, whereas two recent meta-analyses emphasized that immune checkpoint inhibitors related adverse events warranted consideration (24,25). Amounts of clinical trials are ongoing to detect the benefit and risk of PD-1/PD-L1 inhibitors in breast cancer. Pooled analyses of the published results of anti-PD-1/PD-L1 therapies could provide useful information for these ongoing and future explorations in breast cancer. Therefore, in this study, we aim to summarize the antitumor activity and safety of the PD-1/PD-L1 inhibitors in published clinical studies of breast cancer. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-3020).
Methods
Search strategy and study selection
Trials identification followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline (PRISMA) (26).
The search was done in PubMed, Cochrane Library, Web of Science, and EMBASE databases using the terms “nivolumab or pembrolizumab or cemiplimab or atezolizumab or durvalumab or avelumab or PD-1 inhibitor or PD-L1 inhibitor”, “breast cancer or breast neoplasm or breast carcinoma or breast tumor”, and “trial or clinical trial or randomized clinical trial or randomized controlled trial”. We also manually searched the references of relevant published trials and review articles for further eligible studies. The search was completed on Aug 1, 2019.
Studies eligible for inclusion met all of the following criteria: (I) phase I to IV trials in patients with breast cancer, (II) participants were treated with a single agent PD-1/PD-L1 inhibitor or with a combination therapy including a PD-1/PD-L1 inhibitor, (III) inclusion of antitumor activity and safety data, (IV) trials were published in English. Conference abstracts were excluded due to the absence of adverse events data and the increase of heterogeneity. This is because the conference reports are intended to show the positive results rather than negative results. For multiple publications that were identified reporting on the same trial population, the one with the most complete publication data was selected. PD-L1 positive (+) breast cancer was defined as ≥1% tumor cells, lymphocytes, and macrophages. BW and GL reviewed the articles independently. Any discrepancies regarding the literature search, study selection, and data extraction of an article were resolved by discussion.
Data extraction
Detailed reviews of full-text articles were performed by two authors (BW and GL) independently. The first author’s name, publication year, trial name, study design, number of patients, number of TNBC patients, PD-L1 status, phase, cancer type, PD-1 and PD-L1 inhibitor used, and dosing schedule were obtained from each included study. Objective response rate (ORR), disease control rate (DCR), median overall survival (OS), median progression-free survival (PFS), median time to response, median duration of response, and safety data reporting in the publication were collected.
Statistical analysis
All analyses were done using STATA statistical software (version 14.0) and P<0.05 was considered statistically significant. Random-effects models were applied for all pooled effect sizes due to the absence of corresponding single-arm trials. As mean rates could not be smaller than 0, some 95% confidence intervals (CIs) below 0 were considered as 0. Statistical heterogeneity between studies was tested by the Cochran Q chi-square test and I2 statistic percentages, and P<0.10 indicated apparent heterogeneity. I2<50% was defined as low heterogeneity, otherwise was high heterogeneity. Subgroup analyses were performed according to the drug types for ORR, DCR, any-grade and grade ≥3 treatment related adverse events. Egger’s test was used to evaluate latent publication bias for small-study effects.
Results
Eligible studies and characteristics
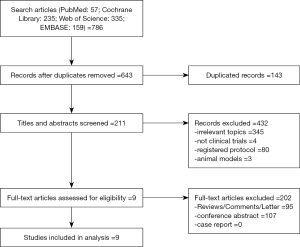
Literature search and review of reference lists identified 786 relevant publications. After screening and eligibility assessment, we included in the systematic review a total of 9 clinical trials involving 1,137 breast cancer patients, comprising one randomized controlled trial (27) and eight single-arm trials (28-35) (Figure 1). The PD-1 and PD-L1 inhibitors used included pembrolizumab (n=5), nivolumab (n=0), cemiplimab (n=0), atezolizumab (n=3), avelumab (n=1), and durvalumab (n=0). Six studies involved the treatment of triple-negative breast cancer (TNBC), two studies included non-TNBC, and one study had both TNBC and non-TNBC arms. The treatment strategy included atezolizumab plus nab-paclitaxel (n=2), pembrolizumab plus trastuzumab (n=1), atezolizumab (n=1), avelumab (n=1), and pembrolizumab (n=4). The primary characteristics of the nine eligible studies were presented in Table 1.
Table 1
| Study [year] | Trial | Phase | Patients | TNBC patients | PD-L1+ patients | Age, mean [range] | Race | Drug | Dose | Clinical setting | Combined with | Line of therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peter Schmid [2018] | IMpassion130 | III | 451 | 451 (100%) | 185 (41.02%) | 55 [20–82] | White, Asian, Black, Native American, Hawaiian or other Pacific Islander, Multiple and Unknown | Atezolizumab | 800 mg | d1 and d15, q4w, iv, minimum 1 cycle | Nab-paclitaxel, 100 mg/m2, d1, 8, 15, q4w, 6 cycles or more | 1 line |
| Sylvia Adams [2018] | GP28328 | Ib | 33 | 33 (100%) | 12 (36.36%) | 55 [32–84] | White, Black or African American, Asian, Mutiple, Other | Atezolizumab | 800 mg | d1 and d15, q2w, iv, minimum 4 cycles | Nab-paclitaxel, 125 mg/m2, d1, 8, 15, q4w, minimum 4 cycles | 1+ line |
| Luc Y. Dirix [2017] | JAVELIN | Ib | 168 | 58 (34.52%) | 85 (50.60%) | 55 [31–81] | White, Black or African American, Asian, Other | Avelumab | 10 mg/kg | d1, q2w, iv, minimum 1 cycle | Single-agent | 2+ line |
| Leisha A. Emens [2018] | PCD4989g | I | 116 | 116 (100%) | 91 (78.45%) | 53 [29–82] | Not mentioned | Atezolizumab | 15 or 20 mg/kg, or at a 1,200-mg flat dose | d1, q3w, iv, minimum 1 cycle | Single-agent | 1+ line |
| Rita Nanda [2016] | KEYNOTE-012 | Ib | 32 | 32 (100%) | 32 (100%) | 50.5 [29–72] | White, Black or African American | Pembrolizumab | 10 mg/kg | d1, q2w, iv, minimum 1 cycle | Single-agent | 1+ line |
| Sylvia Adams [2018] | KEYNOTE-086 cohort A | II | 170 | 170 (100%) | 105 (61.76%) | 53.5 [28–85] | NR | Pembrolizumab | 200 mg | d1, q3w, iv, minimum 1 cycle, up to 2 years | Single-agent | 2+ line |
| Sylvia Adams [2018] | KEYNOTE-086 cohort B | II | 84 | 84 (100%) | 84 (100%) | 52.5 [26–91] | NR | Pembrolizumab | 200 mg | d1, q3w, iv, minimum 1 cycle, up to 2 years | Single-agent | 1 line |
| Sherene Loi [2019] | PANACEA | Ib-II | 58 | 0 (0%) | 46 (79.31%) | NR | NR | Pembrolizumab | Phase Ib: 2 mg/kg or 10 mg/kg; Phase II: 200 mg | d1, q3w, iv, minimum 1 cycle, up to 2 years | Trastuzumab, 6 mg/kg | 1+ line |
| Hope S. Rugo [2018] | KEYNOTE-028 | Ib | 25 | 0 | 25 (100%) | 53 [36–79] | White, Asian, Black or African American, and not specified | Pembrolizumab | 10 mg/kg | d1, q2w, iv, minimum 1 cycle, up to 2 years | Single-agent | 1+ line |
TNBC, triple-negative breast cancer; PD-L1, programmed death-ligand 1; NR, not reported.
PFS and OS
Table 2 displayed the main survival outcomes in the selected studies. In IMpassion130 trial, previously untreated metastatic TNBC patients received nab-paclitaxel plus atezolizumab or placebo. The median PFS was 7.2 months in the atezolizumab group, as compared with 5.5 months in the placebo group. The median OS was 21.3 months in the atezolizumab group and 17.6 months in the placebo group. In PD-L1+ patients treated with atezolizumab plus nab-paclitaxel, the median PFS and OS were, respectively, 7.5 and 25 months. Recurrent or metastatic TNBC patients in GP28328 trial were similarly treated with atezolizumab plus nab-paclitaxel. But the median PFS and OS were decreased to 5.5 and 14.7 months. In PD-L1+ population, the median PFS and OS were decreased to 6.9 and 21.9 months. The differences between the two studies might be attributed to the lines of prior systemic chemotherapy, as patients in GP28328 had received several lines of previous chemotherapy.
Table 2
| Trial | Median follow-up (months) | Median TTR (months) | Median DOR (months) | Median PFS (months) | Median OS (months) | PD-L1+Median TTR (months) | PD-L1+Median DOR (months) | PD-L1+Median PFS (months) | PD-L1+Median OS (months) |
|---|---|---|---|---|---|---|---|---|---|
| IMpassion130 | 12.9 | NR | 7.4 (95% CI, 6.9–9.0) | 7.2 (95% CI, 5.6–7.5) |
21.3 (95% CI, 17.3–23.4) | NR | 8.5 (95% CI, 7.3–9.7) | 7.5 (95% CI, 6.7–9.2) |
25 (95% CI, 22.6–not estimable) |
| GP28328 | 24.4 (95% CI, 22.1–28.8) |
NR | 9.1 (95% CI, 2.0–20.9) | 5.5 (95% CI, 5.1–7.7) |
14.7 (95% CI, 10.1–not estimable) | NR | 9.1 (95% CI, 2.9–16.2) | 6.9 (95% CI, 5.2–11.0) |
21.9 (95% CI, 13.1–not estimable) |
| JAVELIN | 10 (range, 6.0–15.2) |
2.7 (range, 1.3–4.1) |
Not estimable (95% CI, 6.7–not estimable) | 1.4 (95% CI, 1.4–1.4) |
8.1 (95% CI, 6.4–not estimable) | NR | NR | 1.4 months (95% CI, 1.3–1.4) |
6.5 (95% CI, 3.7–9.2) |
| PCD4989g | 25.3 (range, 0.4–45.6) |
NR | 21 (range, 3 to ≥38) | 1.4 (95% CI, 1.3–1.6) |
8.9 (95% CI, 7.0–12.6) | NR | NR | 1.4 (95% CI, 1.3–1.9) |
10.1 (95% CI, 7.0–13.8) |
| KEYNOTE-012 | 10.0 (range, 0.4–19.5) | 4.2 (range, 1.7–7.6) |
Not estimable (95% CI, 3.5 to ≥11.0) |
1.9 (95% CI, 1.7–5.5) |
11.2 (95% CI, 5.3–not estimable) | 4.2 (range, 1.7–7.6) |
Not estimable (95% CI, 3.5 to ≥11.0) | 1.9 months (95% CI, 1.7–5.5) |
11.2 (95% CI, 5.3–not estimable) |
| KEYNOTE-086 cohort A | 9.6 (range, 0.1–25.7) |
3.9 (range, 1.9–8.1) |
Not estimable (95% CI, ≥1.2 to ≥21.5) |
2.0 (95% CI, 1.9–2.0) |
9.0 (95% CI, 7.7–11.2) | 3.1 (range, 1.9–6.2) |
Not estimable (95% CI, 6.3 to ≥21.5) | 2.0 (95% CI, 1.9–2.1) |
8.8 (95% CI, 7.1–11.2) |
| KEYNOTE-086 cohort B | 12.3 (range, 0.9–23.5) |
2.0 (range, 1.7–6.2) |
10.4 (95% CI, 4.2 to ≥19.2) |
2.1 (95% CI, 2.0–2.2) |
18.0 (95% CI, 12.9–23.0) | 2.0 (range, 1.7–6.2) |
10.4 (95% CI, 4.2 to ≥19.2) | 2.1 (95% CI, 2.0–2.2) |
18.0 (95% CI, 12.9–23.0) |
| PANACEA | Phase Ib: 25.7 (IQR, 25.6–25.8); phase II: 13.6 (IQR, 11.6–18.4) | NR | NR | NR | NR | 2.7 (95% CI, 2.6–4.0) |
3.5 (95% CI, 2.7–not estimable) | 2.7 (95% CI, 2.6–4.0) |
Not estimable (95% CI, 13.1–not estimable) |
| KEYNOTE-028 | 9.7 (range, 0.7-31.8) | 1.7 (range, 1.7–1.9) |
12.0 (range, 7.4–15.9) | 1.8 (95% CI, 1.4–2.0) |
8.6 (95% CI, 7.3–11.6) | 1.7 (range, 1.7–1.9) |
12.0 (range, 7.4–15.9) | 1.8 (95% CI, 1.4–2.0) |
8.6 (95% CI, 7.3–11.6) |
TTR, time to response; DOR, duration of response; PFS, progression-free survival; OS, overall survival; 95% CI, 95% confidence interval; NR, not reported.
However, in PCD4989g trial, TNBC patients treated with atezolizumab monotherapy had a median PFS of 1.4 months and a median OS of 8.9 months. Ninety-one (79.1%) of 115 participants were PD-L1+ breast cancer. In this cohort, the median OS prolonged 1.2 months but not PFS. Avelumab showed a similar efficacy on breast cancer including TNBC and non-TNBC. Nevertheless, the median OS was 6.5 months in PD-L1+ patients.
The median PFS of pembrolizumab treated PD-L1+ TNBC patients ranged from 1.9 to 2.1 months, while the median OS ranged from 8.8 to 18 months. Additionally, patients with PD-L1+ non-TNBC showed a median PFS of 1.8 months and a median OS of 8.6 months after the pembrolizumab treatment. When PD-L1+ non-TNBC patients were administrated with pembrolizumab plus trastuzumab, the median PFS was 2.7 months, with an unreached median OS.
ORR
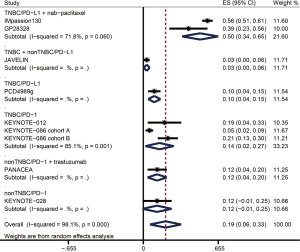
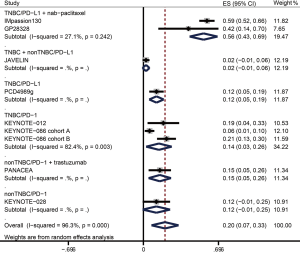
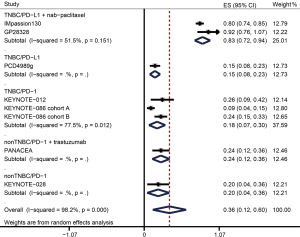
The ORR data were available from nine trials including 1,130 patients in overall population and 660 patients in PD-L1+ population (Table 3). Figure 2 and Figure 3 showed the pooled ORRs for overall population and PD-L1+ population respectively. In TNBC patients received anti-PD-L1 plus nab-paclitaxel therapy, the pooled ORR was 49.7% (95% CI: 33.9–65.5%) in overall population, and 55.8% (95% CI: 42.9–68.6%) in PD-L1+ population. The ORR of anti-PD-L1 monotherapy in TNBC was 9.6% (95% CI: 4.2–15.0%) in overall population and 12.1% (95% CI: 5.4–18.8%) in PD-L1+ population. In the anti-PD-L1 treatment for breast cancer containing both TNBC and non-TNBC, the ORRs of overall and PD-L1+ cohort were 3.0% (95% CI: −0.4–5.6%) and 2.4% (95% CI: −0.9–5.7%). The pooled ORR for PD-L1+ TNBC patients administrated with a PD-1 inhibitor was 14.4% (95% CI: 2.5–26.3). PD-L1+ non-TNBC patients received anti-PD-1 therapy had an ORR with 12.0% (−0.7–24.7%). When non-TNBC patients were treated with anti-PD-1 plus trastuzumab regimen, the ORRs were 12.1% (95% CI: 3.7–20.5%) in overall population and 15.2% (95% CI: 4.8–25.6%) in PD-L1+ population.
Table 3
| Study | Overall | PD-L1 positive | |||||
|---|---|---|---|---|---|---|---|
| n | MR | 95% CI | n | MR | 95% CI | ||
| TNBC/anti-PD-L1 + nab-paclitaxel | |||||||
| IMpassion130 | 450 | 0.560 | 0.514–0.606 | 185 | 0.589 | 0.518–0.660 | |
| GP28328 | 33 | 0.394 | 0.227–0.561 | 12 | 0.417 | 0.138–0.696 | |
| Sub-total | 483 | 0.497 | 0.339–0.655 | 197 | 0.558 | 0.429–0.688 | |
| TNBC + non-TNBC/Anti-PD-L1 | |||||||
| JAVELIN | 168 | 0.030 | 0.004–0.056 | 85 | 0.024 | −0.009–0.057 | |
| TNBC/anti-PD-L1 | |||||||
| PCD4989g | 115 | 0.096 | 0.042–0.150 | 91 | 0.121 | 0.054–0.188 | |
| TNBC/anti-PD-1 | |||||||
| KEYNOTE-012 | 27 | 0.185 | 0.039–0.331 | 27 | 0.185 | 0.039–0.331 | |
| KEYNOTE-086 cohort A | 170 | 0.053 | 0.019–0.087 | 105 | 0.057 | 0.013–0.101 | |
| KEYNOTE-086 cohort B | 84 | 0.214 | 0.126–0.302 | 84 | 0.214 | 0.126–0.302 | |
| Sub-total | 281 | 0.142 | 0.018–0.266 | 216 | 0.144 | 0.025–0.263 | |
| Non-TNBC/Anti-PD-1 + trastuzumab | |||||||
| PANACEA | 58 | 0.121 | 0.037–0.205 | 46 | 0.152 | 0.048–0.256 | |
| Non-TNBC/anti-PD-1 | |||||||
| KEYNOTE-028 | 25 | 0.120 | −0.007–0.247 | 25 | 0.120 | −0.007–0.247 | |
ORR, objective response rate; TNBC, triple-negative breast cancer; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; n, number of patients; MR, mean rate; CI, confidence interval.
DCR
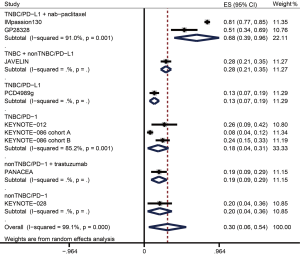
A total of 1,130 patients from nine studies were analyzed in the pooled DCR of overall population, and 575 patients from eight studies were analyzed in the pooled DCR of PD-L1+ population (Table 4). Figure 4 and Figure 5 showed the pooled DCRs for overall population and PD-L1+ population respectively. The pooled DCRs of overall population and PD-L1+ population for anti-PD-L1 + nab-paclitaxel treated TNBC patients were 67.5% (95% CI: 38.6–96.4%) and 83.4% (95% CI: 72.2–94.5%). In the overall group, the pooled DCR of anti-PD-L1 therapy for TNBC was 13.0% (95% CI: 6.9–19.1%) versus 28.0% (95% CI: 21.2–34.8%) in TNBC + non-TNBC subgroup. In the group of PD-L1+ patients, the pooled DCR of anti-PD-L1 therapy for TNBC was 15.4% (95% CI: 8.0–22.8%). Anti-PD-1 therapy had a pooled DCR with 18.4% (95% CI: 6.8–30.1%) for PD-L1+ TNBC cohort. PD-L1+ non-TNBC patients had a DCR of 20% (95% CI: 4.3–35.7%) in anti-PD-1 treatment versus 23.9% (95% CI: 11.6–36.2%) in anti-PD-1 plus trastuzumab treatment. When overall non-TNBC patients were treated with anti-PD-1 plus trastuzumab, the DCR was 19.0% (8.9–29.1%).
Table 4
| Study | Overall | PD-L1 positive | |||||
|---|---|---|---|---|---|---|---|
| n | MR | 95% CI | n | MR | 95% CI | ||
| TNBC/Anti-PD-L1 + nab-paclitaxel | |||||||
| IMpassion130 | 450 | 0.811 | 0.775–0.847 | 185 | 0.795 | 0.737–0.853 | |
| GP28328 | 33 | 0.515 | 0.344–0.686 | 12 | 0.917 | 0.761–1.073 | |
| Sub-total | 483 | 0.675 | 0.386–0.964 | 197 | 0.834 | 0.722–0.945 | |
| TNBC + non-TNBC/anti-PD-L1 | |||||||
| JAVELIN | 168 | 0.280 | 0.212–0.348 | – | – | – | |
| TNBC/anti-PD-L1 | |||||||
| PCD4989g | 115 | 0.130 | 0.069–0.191 | 91 | 0.154 | 0.080–0.228 | |
| TNBC/anti-PD-1 | |||||||
| KEYNOTE-012 | 27 | 0.259 | 0.094–0.424 | 27 | 0.259 | 0.094–0.424 | |
| KEYNOTE-086 cohort A | 170 | 0.076 | 0.036–0.116 | 105 | 0.095 | 0.039–0.151 | |
| KEYNOTE-086 cohort B | 84 | 0.238 | 0.147–0.329 | 84 | 0.238 | 0.147–0.329 | |
| Sub-total | 281 | 0.179 | 0.044–0.313 | 216 | 0.184 | 0.068–0.301 | |
| Non-TNBC/anti-PD-1 + trastuzumab | |||||||
| PANACEA | 58 | 0.190 | 0.089–0.291 | 46 | 0.239 | 0.116–0.362 | |
| Non-TNBC/an-PD-1 | |||||||
| KEYNOTE-028 | 25 | 0.200 | 0.043–0.357 | 25 | 0.200 | 0.043–0.357 | |
DCR, disease control rate; TNBC, triple-negative breast cancer; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; n, number of patients; MR, mean rate; CI, confidence interval.
Treatment related adverse events
Of 1,080 breast cancer patients from eight trials, 901 (83.43%) developed at least 1 treatment related adverse event of any grade, and 394 (34.62%) of 1,138 from nine trials developed at least 1 grade ≥3 treatment related adverse event (Table 5).
Table 5
| Study | Any-grade | Grade ≥3 | |||||
|---|---|---|---|---|---|---|---|
| n | MR | 95% CI | n | MR | 95% CI | ||
| TNBC/anti-PD-L1 + nab-paclitaxel | |||||||
| IMpassion130 | 452 | 0.993 | 0.986–1.001 | 452 | 0.487 | 0.441–0.533 | |
| GP28328 | – | – | – | 33 | 0.727 | 0.575–0.879 | |
| Sub-total | – | – | – | 485 | 0.596 | 0.361–0.830 | |
| TNBC + non-TNBC/anti-PD-L1 | |||||||
| JAVELIN | 168 | 0.685 | 0.614–0.755 | 168 | 0.137 | 0.085–0.189 | |
| TNBC/anti-PD-L1 | |||||||
| PCD4989g | 116 | 0.983 | 0.959–1.006 | 116 | 0.509 | 0.418–0.600 | |
| TNBC/anti-PD-1 | |||||||
| KEYNOTE-012 | 32 | 0.563 | 0.391–0.734 | 32 | 0.156 | 0.030–0.282 | |
| KEYNOTE-086 cohort A | 170 | 0.606 | 0.532–0.679 | 170 | 0.129 | 0.079–0.180 | |
| KEYNOTE-086 cohort B | 84 | 0.631 | 0.528–0.734 | 84 | 0.095 | 0.032–0.158 | |
| Sub-total | 286 | 0.609 | 0.552–0.665 | 286 | 0.120 | 0.082–0.157 | |
| Non-TNBC/anti-PD-1 + trastuzumab | |||||||
| PANACEA | – | – | – | 58 | 0.500 | 0.371–0.629 | |
| Non-TNBC/an-PD-1 | |||||||
| KEYNOTE-028 | 25 | 0.640 | 0.452–0.828 | 25 | 0.160 | 0.016–0.304 | |
TNBC, triple-negative breast cancer; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; n, number of patients; MR, mean rate; CI, confidence interval.
The incidences of any-grade and grade ≥3 treatment related adverse events in PD-1 inhibition plus nab-paclitaxel treated TNBC were 99.3% (95% CI: 98.6–100.1%) and 59.6% (95% CI: 36.1–83.0%). In anti-PD-L1 monotherapy, the incidence of any-grade treatment related adverse events was 98.3% (95% CI: 95.9–100.6%) in TNBC versus 68.5% (95% CI: 61.4–75.5%) in breast cancer containing TNBC and non-TNBC. The incidences of any-grade and grade ≥3 treatment related adverse events in TNBC patients received anti-PD-1 therapy were 60.9% (95% CI: 55.2–66.5%) and 12.0% (95% CI: 8.2–15.7%). In addition, the incidence of grade ≥3 treatment related adverse events in non-TNBC were 16.0% (95% CI: 1.6–30.4%) in anti-PD-1 monotherapy versus 50.0% (95% CI: 37.1–62.9%) in anti-PD-1 plus trastuzumab therapy.
As shown in Table 6 and Table 7, we focused on treatment related adverse events that were reported by at least three studies. Using the criteria, the most common any-grade treatment related adverse events were fatigue (29.2%, 95% CI: 15.8–42.6%), nausea (20.2%, 95% CI: 9.7–30.7%), neutropenia (19.4%, 95% CI: 7.5–31.3%), and diarrhea (16.8%, 95% CI: 8.7–25.0%) (Table 6). The most common grade ≥3 treatment related adverse events were neutropenia (6.0%, 95% CI: 1.0–10.9%), anemia (2.3%, 95% CI: 1.3–3.2%), diarrhea (1.4%, 95% CI: 0.6–2.3%), and dyspnea (1.0%, 95% CI: −0.3–2.3%) (Table 7).
Table 6
| Toxicities | n | MR | 95% CI |
|---|---|---|---|
| Fatigue | 1,138 | 0.292 | 0.158–0.426 |
| Nausea | 1,138 | 0.202 | 0.097–0.307 |
| Neutropenia | 769 | 0.194 | 0.075–0.313 |
| Diarrhea | 1,113 | 0.168 | 0.087–0.250 |
| Dyspnea | 678 | 0.135 | 0.022–0.247 |
| Anemia | 911 | 0.134 | 0.038–0.229 |
| Headache | 658 | 0.130 | 0.022–0.238 |
| Rash | 797 | 0.108 | 0.042–0.173 |
| Arthralgia | 1,054 | 0.105 | 0.057–0.153 |
| Vomiting | 710 | 0.104 | 0.030–0.178 |
| Pruritus | 909 | 0.098 | 0.061–0.135 |
| Hypothyroidism | 990 | 0.079 | 0.040–0.118 |
| Infusion-related reaction | 422 | 0.050 | −0.002–0.102 |
| ALT increased | 404 | 0.042 | 0.014–0.070 |
| AST increased | 407 | 0.035 | 0.017–0.053 |
| Hyperthyroidism | 422 | 0.032 | −0.004–0.068 |
| Pneumonia | 513 | 0.028 | 0.014–0.042 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; n, number of patients; MR, mean rate; CI, confidence interval.
Table 7
| Toxicities | n | MR | 95% CI |
|---|---|---|---|
| Neutropenia | 769 | 0.060 | 0.010–0.109 |
| Anemia | 943 | 0.023 | 0.013–0.032 |
| Diarrhea | 739 | 0.014 | 0.006–0.023 |
| Dyspnea | 678 | 0.010 | −0.003–0.023 |
| Vomiting | 626 | 0.009 | 0.002–0.017 |
| Nausea | 647 | 0.009 | 0.002–0.017 |
| Fatigue | 874 | 0.008 | 0.002–0.014 |
| AST increased | 259 | 0.008 | −0.003–0.019 |
| ALT increased | 256 | 0.008 | −0.003–0.019 |
| Pneumonia | 429 | 0.007 | −0.001–0.015 |
| Arthralgia | 769 | 0.003 | −0.001–0.007 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; n, number of patients; MR, mean rate; CI, confidence interval.
Heterogeneity and publication bias
Even a random-effects model was applied for all pooled data analysis and subgroup analyses were conducted, heterogeneity was high owing to the eligible studied in our analysis were almost phase I and II trials. Additionally, publication bias was not observed in the results of Egger’s test based on the analysis of ORR (overall: P=0.393>0.05; PD-L1+: P=0.191>0.05) and DCR (overall: P=0.466>0.05; PD-L1+: P=0.973>0.05).
Discussion
This study quantitatively integrated the results of published clinical trials and was conducted to estimate the antitumor activity and safety of PD-1/PD-L1 inhibitors in patients with breast cancer.
In PD-L1 positive breast cancer patients treated with PD-L1 inhibitors (atezolizumab and avelumab), the pooled ORRs ranged from 2.4% in JAVELIN to 58.9% in IMpassion130. The difference mainly caused by two reasons: first, patients in IMpassion130 were previously untreated, whereas patients in JAVELIN had received prior lines of cytotoxic therapy; second, atezolizumab was used in IMpassion130, and avelumab was used in JAVELIN. In the phase Ib trial GP28328, although patients were also received previous systemic cytotoxic regimens, the ORR was 41.7% in PD-L1+ population. Additionally, when patients were treated with single atezolizumab agent, the ORR of PD-L1 positive patients was 12.1% in PCD4989g study. Studies of combination treatment that might increase the probability of antitumor activity were warranted, and promising treatment benefit in TNBC had been reported for a treatment regimen of pembrolizumab in combination with eribulin mesylate and of atezolizumab administered in combination with taxane chemotherapy in preliminary studies (36,37). In our analysis, patients in both IMpassion130 and GP28328 had received atezolizumab plus nab-paclitaxel therapy and the rates of progressive disease were sharply decreased (15.3% in IMpassion130 and 18.2% in GP28328). Based on the presence of TILs in tumor tissues, TNBC were immunogenic and higher percentages of TILs were relevant to response to PD-1/PD-L1 inhibitors (30,38). In addition, cytotoxic drugs might enhance the efficacy of immunotherapy via increasing the expression of PD-L1 (39). Thus, we supposed that atezolizumab combined with nab-paclitaxel could be an option of front-line therapeutic paradigm for advanced or metastatic breast cancer. Moreover, PD-L1 positive breast cancer patients might have higher responses when receiving anti-PD-L1 therapy plus cytotoxic treatment.
Recently, there are several ongoing clinical trials in studying the combination therapy of a PD-1/PD-L1 inhibitor and chemotherapy. IMpassion031 is comparing neoadjuvant atezolizumab vs placebo in combination with anthracycline/nab-paclitaxel-based chemotherapy in early TNBC (40). IMpassion132 is evaluating atezolizumab with first-line chemotherapy [capecitabine (mandatory in platinum-pretreated patients) or gemcitabine/carboplatin] for inoperable locally advanced/metastatic TNBC (41). Moreover, KEYNOTE-355 is a global phase III study of pembrolizumab + chemotherapy (pembrolizumab + nab-paclitaxel, pembrolizumab +paclitaxel, pembrolizumab +gemcitabine/carboplatin) vs. placebo + chemotherapy in patients with previously untreated, locally recurrent, inoperable TNBC (42). KEYNOTE-522 is a phase III study of pembrolizumab + chemotherapy vs. placebo + chemotherapy as neoadjuvant treatment, followed by pembrolizumab vs. placebo as adjuvant treatment in patients with TNBC (43). Additionally, we noticed that the median time to response in KEYNOTE-028 was 1.7 months (range, 1.9–1.9 months). However, in KEYNOTE-012, the median time to response was 17.9 weeks (range, 7.3–32.4 weeks). Both the trails were taken single agent without combination with chemotherapy. As the progressive disease rate in KEYNOTE-012 was 48.1% and in KEYNOTE-028 was 60.0%, the long time to response might be a critical reason for the high rate of progressive disease. Taken together, the data for anti-PD-L1 agents appeared encouraging for patients with PD-L1 positive breast cancer and showed that PD-L1 might be a predictor of response to PD-L1 antagonists. Further, anti-PD-1/PD-L1 agents plus chemotherapy could achieve more efficacy than expected. Thus, in the future researches of PD-1/PD-L1 in breast cancer, if anti-PD-1/PD-L1 monotherapy fails to exert expected effects, combination therapeutic strategies could be another choice (44).
From the standpoint of patient counseling, several results of adverse events are important. Approximately 83.43% breast cancer patients treated with PD-1/PD-L1 inhibitors in clinical trials experienced at least 1 treatment related adverse event of any grade, and 34.62% breast cancer patients had at least 1 grade ≥3 treatment related adverse event. Moreover, PD-L1 inhibitors had a higher incidence of any-grade and grade ≥3 treatment related adverse events than PD-1 inhibitors in TNBC (any-grade: 98.3% vs. 60.9%; grade ≥3: 50.9% vs. 12.0%). These numbers can be important to share with patients with breast cancer before they begin treatment with an anti-PD-1/PD-L1 agent. Fatigue was the most common any-grade treatment related adverse event (29.2%), and neutropenia was the most common grade ≥3 treatment related adverse event (6.0%). Nausea, neutropenia and diarrhea are the next most common any-grade treatment related adverse events (>15%). Thus, clinical vigilance is needed for early recognition and intervention to prevent severe complications.
Limitations
This study has several limitations. First, the present analysis, including only one randomized controlled trial, was limited as the included studies were all single-arm phase I-II clinical trials. Second, published clinical trials of nivolumab, cemiplimab and durvalumab were absent. Third, since nab-paclitaxel was administrated in IMpassion 130 and GP28328 trials, hematological toxicities, such as neutropenia, might be mainly caused by chemotherapy. Despite the limitations, this analysis is a meaningful study of the estimates of the antitumor activity and safety of PD-1/PD-L1 antagonists.
In conclusion, we found that PD-1 and PD-L1 inhibitors appeared to be effective for treating advanced breast cancer, and anti-PD-L1 plus systemic chemotherapy might be a front- or first-line treatment option for patients with PD-L1 positive advanced TNBC. Meanwhile, careful monitoring of the adverse events of anti-PD-1/PD-L1 agents should be needed. More randomized clinical studies are warranted to confirm our findings.
Acknowledgments
We thank the help of other members in Zhao’s workgroup and the SNOWELL STUDIO.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-3020
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-3020). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005;10:20-9. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:310-20. [Crossref] [PubMed]
- Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 2019;393:2591-8. [Crossref] [PubMed]
- Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427-38. [PubMed]
- Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmgenomics Pers Med 2014;7:203-15. [PubMed]
- Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010;376:235-44. [Crossref] [PubMed]
- Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011;12:852-61. [Crossref] [PubMed]
- Lee JM, Peer CJ, Yu M, et al. Sequence-Specific Pharmacokinetic and Pharmacodynamic Phase I/Ib Study of Olaparib Tablets and Carboplatin in Women's Cancer. Clin Cancer Res 2017;23:1397-406. [Crossref] [PubMed]
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [Crossref] [PubMed]
- Anders CK, Abramson V, Tan T, et al. The Evolution of Triple-Negative Breast Cancer: From Biology to Novel Therapeutics. Am Soc Clin Oncol Educ Book 2016;35:34-42. [Crossref] [PubMed]
- Andre F, Zielinski CC. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol 2012;23:vi46-51. [Crossref] [PubMed]
- Li X, Shao C, Shi Y, et al. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol 2018;11:31. [Crossref] [PubMed]
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol 2018;8:86. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- AiErken N. High PD-L1 Expression Is Closely Associated With Tumor-Infiltrating Lymphocytes and Leads to Good Clinical Outcomes in Chinese Triple Negative Breast Cancer Patients. Int J Biol Sci 2017;13:1172-9. [Crossref] [PubMed]
- Botti G, Collina F, Scognamiglio G, et al. Programmed Death Ligand 1 (PD-L1) Tumor Expression Is Associated with a Better Prognosis and Diabetic Disease in Triple Negative Breast Cancer Patients. Int J Mol Sci 2017;18:459. [Crossref] [PubMed]
- Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014;2:361-70. [Crossref] [PubMed]
- Zhu X, Zhang Q, Wang D, et al. Expression of PD-L1 Attenuates the Positive Impacts of High-level Tumor-infiltrating Lymphocytes on Prognosis of Triple-negative Breast Cancer. Cancer Biol Ther 2019;20:1105-12. [Crossref] [PubMed]
- Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014;20:2773-82. [Crossref] [PubMed]
- Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol 2015;26:1488-93. [Crossref] [PubMed]
- Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015;6:5449-64. [Crossref] [PubMed]
- Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721-8. [Crossref] [PubMed]
- Wang Y, Zhou S, Yang F, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:1008-19. [Crossref] [PubMed]
- Stewart LA, Clarke M, Rovers M, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015;313:1657-65. [Crossref] [PubMed]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
- Adams S, Diamond JR, Hamilton E, et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up A Phase 1b Clinical Trial. JAMA Oncol 2019;5:334-42. [Crossref] [PubMed]
- Dirix LY. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018;167:671-86. [Crossref] [PubMed]
- Emens LA, Cruz C, Eder JP, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer A Phase 1 Study. JAMA Oncol 2019;5:74-82. [Crossref] [PubMed]
- Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34:2460-7. [Crossref] [PubMed]
- Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:397-404. [Crossref] [PubMed]
- Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:405-11. [Crossref] [PubMed]
- Loi S, Giobbie-Hurder A, Gombos A, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol 2019;20:371-82. [Crossref] [PubMed]
- Rugo HS, Delord JP, Im SA, et al. Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer. Clin Cancer Res 2018;24:2804-11. [Crossref] [PubMed]
- Tolaney SM, Kalinsky K, Kaklamani V, et al. Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Cancer Res 2018;78:Abstract nr PD6-13.
- Adams S, Diamond JR, Hamilton EP, et al. Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple negative breast cancer (mTNBC). J Clin Oncol 2016;34:1009. [Crossref]
- Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 2015;26:1698-704. [Crossref] [PubMed]
- Feng D, Qin B, Pal K, et al. BRAF(V600E)-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene 2019;38:6752-66. [Crossref] [PubMed]
- Mittendorf EA, Barrios CH, Harbeck N, et al. IMpassion031: A phase III study comparing neoadjuvant atezolizumab (atezo) vs placebo in combination with anthracycline/nab-paclitaxel (nab-pac)-based chemotherapy in early triplenegative breast cancer (eTNBC). Ann Oncol 2017;28:v65. [Crossref]
- Dent R, Andre F, Goncalves A, et al. IMpassion132: A double-blind randomized phase 3 trial evaluating chemotherapy (CT) +/- atezolizumab (atezo) for early progressing locally advanced/metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 2018;36:TPS1115. [Crossref]
- Cortes J, Guo Z, Karantza V, et al. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (PBO) + chemo for previously untreated, locally recurrent, inoperable or metastatic triplenegative breast cancer (mTNBC). J Clin Oncol 2018;36:TPS18. [Crossref]
- Schmid P, Cortes J, Bergh JCS, et al. KEYNOTE-522: Phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo + chemo as neoadjuvant therapy followed by pembro vs placebo as adjuvant therapy for triple-negative breast cancer (TNBC). J Clin Oncol 2018;36:TPS602. [Crossref]
- Telli ML, Vinayak S. Future of checkpoint blockade in triple-negative breast cancer: Combination strategies to lead the way. Ann Oncol 2019;30:347-8. [Crossref] [PubMed]