
New cancers therapy through targeting Aldo-keto reductase family 1 member B10 with miRNAs
Aldo-keto reductase family 1 member B10 (AKR1B10), also known as aldose reductase-like-1 (ARL-1), mainly reduces substrates such as retinaldehyde and lipid peroxidation products. Accumulating evidence indicates that AKR1B10 is an oncogene and contributes to human cancers, such as breast, lung and liver cancer (1-3). AKR1B10 is a biomarker and treatment target of cancer. Numbers of AKR1B10 inhibitors, such as aldose reductase inhibitors (ARIs), endogenous substances, natural-based derivatives, synthetic compounds, could be novel anticancer drugs (4). Thus, targeting AKR1B10 may be a way of cancer therapy.
Recently, Wang and colleagues indicated that AKR1B10 promoted hepatocellular carcinoma cell growth and degenerated by miR-383-5p (5). The authors initially analyzed GEO microarray and TCGA RNAseq dataset, and identified AKR1B10 serving as an oncogene in HCC. Then, they confirmed that AKR1B10 promotes HCC tumor growth in vitro. AKR1B10 expression was found to be negatively to miR-383-5p. More additional, the survival analysis showed that high expression of AKR1B10 is associated with poor prognosis, and low expression of miR-383-5p is associated with poor prognosis. Mechanistically, miR-383-5p was found to directly target 3'-UTR of AKR1B10 mRNA, and degenerate AKR1B10.
Cong et al. demonstrated that linc00665 could promote lung adenocarcinoma (LUAD) development and act as ceRNA to regulate AKR1B10-ERK signaling by sponging miR-98 (3). The authors found that linc00665 was significantly unregulated LUAD tissues, and linc00665 might be an independent predictor for poor prognosis of LUAD. Linc00665 promoted LUAD cell proliferation and metastasis both in vitro and in vivo. Both linc00665 and AKR1B10 could bind to miR-98. Linc00665 interacted with miR-98, and then liberated AKR1B10 mRNA transcripts, subsequently stimulating the downstream AKR1B10-ERK signaling pathway. They confirmed our findings in which AKR1B10 activated cancer cell migration and invasion through stimulating ERK signaling. Thus, these results suggested that AKR1B10-ERK signaling pathway plays important role in cancer development.
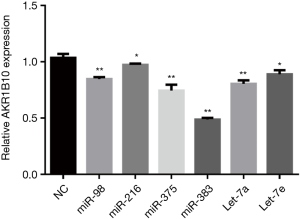
We then predicted the potential target miRNAs of AKR1B10 using the online bioinformatics tools microrna (www.microrna.org). We found more potential miRNAs, which might bind to AKR1B10 UTR (Figure 1). Mir-216 and miR-375 were identified as tumor suppressors in a variety of cancers (6-8). We inferred that mir-216 and miR-375 may also target AKR1B10 and post-transcriptional regulate AKR1B10 expression. We performed qRT-PCR assay to verify our predictions. Results showed that AKR1B10 expression was decreased after overexpression potential target miRNAs in A549 cell line, which suggested that AKR1B10 is regulated by these miRNAs (Figure 2). Taking together, these studies expand our knowledge of AKR1B10 post-transcriptional regulation by miRNAs. Targeting AKR1B10 with miRNAs will be a new treatment of cancer in the future.
Acknowledgments
Funding: This work was supported by
Footnote
Provenance and Peer Review: This article was a standard submission to the editorial office, Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2467). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shi J, Chen L, Chen Y, et al. Aldo-Keto Reductase Family 1 Member B10 (AKR1B10) overexpression in tumors predicts worse overall survival in hepatocellular carcinoma. J Cancer 2019;10:4892-901. [Crossref] [PubMed]
- Ye X, Li C, Zu X, et al. A Large-Scale Multicenter Study Validates Aldo-Keto Reductase Family 1 Member B10 as a Prevalent Serum Marker for Detection of Hepatocellular Carcinoma. Hepatology 2019;69:2489-501. [PubMed]
- Cong Z, Diao Y, Xu Y, et al. Long non-coding RNA linc00665 promotes lung adenocarcinoma progression and functions as ceRNA to regulate AKR1B10-ERK signaling by sponging miR-98. Cell Death Dis 2019;10:84. [Crossref] [PubMed]
- Huang L, He R, Luo W, et al. Aldo-Keto Reductase Family 1 Member B10 Inhibitors: Potential Drugs for Cancer Treatment. Recent Pat Anticancer Drug Discov 2016;11:184-96. [Crossref] [PubMed]
- Wang J, Zhou Y, Fei X, et al. Biostatistics mining associated method identifies AKR1B10 enhancing hepatocellular carcinoma cell growth and degenerated by miR-383-5p. Sci Rep 2018;8:11094. [Crossref] [PubMed]
- Sun Y, Hu B, Wang Y, et al. miR-216a-5p inhibits malignant progression in small cell lung cancer: involvement of the Bcl-2 family proteins. Cancer Manag Res 2018;10:4735-45. [Crossref] [PubMed]
- Zhang Y, Lin P, Zou JY, et al. MiR-216a-5p act as a tumor suppressor, regulating the cell proliferation and metastasis by targeting PAK2 in breast cancer. Eur Rev Med Pharmacol Sci 2019;23:2469-75. [PubMed]
- Xu X, Chen X, Xu M, et al. miR-375-3p suppresses tumorigenesis and partially reverses chemoresistance by targeting YAP1 and SP1 in colorectal cancer cells. Aging (Albany NY) 2019;11:7357-85. [Crossref] [PubMed]



