
Multidisciplinary team approach on a case of bronchopleural fistula after video-assisted thoracoscopic segmentectomy: a case report
Introduction
Bronchopleural fistulas (BPFs) are one of the most severe post-surgery complications associated with lung diseases. Their rapid progress and high mortality remain a major challenge for thoracic surgeons. The prognosis and treatment difficulty of BPF differ from the different resection range of lung tissue. BPF secondary to pneumonectomy has the most difficult treatment and the highest mortality due to its severe thoracic and systemic infection. BPF located on the stump of segmental bronchi after segmental bronchi after segmental resection is relatively small, and the success rate of conservative treatment is higher than pneumonectomy and lobectomy due to localized thoracic infection. With the progressive advance of clinical diagnosis and treatment techniques, treatment of BPFs has made dramatic progress, and the success rate of treatment has significantly improved. Herein, we report a case study of a patient with BPF after segmental resection. After a multidisciplinary discussion, conservative drainage therapy was successfully performed to avoid traumatic surgical repair. We present the following case in accordance with the CARE reporting (available at http://dx.doi.org/10.21037/tcr-19-1930).
Case presentation
Patient information
The patient was a 41-year-old male admitted to the Department of Thoracic Surgery because of right lung nodules 7 days prior. He underwent a posterior segmentectomy of the right upper lobe and posterolateral basal segmentectomy of the right lower lobe through video-assisted thoracoscopy. The patient recovered well after surgery. Three days after discharge the patient began to have a cough with malodorous yellow phlegm accompanied by fever, reaching a 39 °C body temperature. The patient was immediately admitted with a primary diagnosis of lung infection.
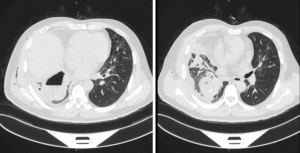
The patient denied a history of hypertension, coronary heart disease, tuberculosis, smoking, and drinking. The patient had no pastoral, mining, or chemical exposure history. Physical examination revealed the following: pulse 80 beats/min, body temperature 38.5 °C, breathing 12 beats/min, blood pressure 130/70 mmHg, and weight 78 kg. The right chest wall had crepitus according to touch. The sound of the lung breathing was thick, there were no obvious dry or wet rales, and there was a regular cardiac rhythm with no obvious pathological murmur. Chest computed tomography (CT) imaging showed that pleural effusion and gas accumulation were visible in the right thoracic cavity, the residual cavity was formed, the two lung lobes were blurred, and the right pleura was thickened (Figure 1). The blood work suggested that the white blood cell count was 25.71×109/L, and the proportion of neutrophils was 84.2%.
Diagnosis, treatment and clinical outcome
After admission, the patient was administered cefoperazone sulbactam combined with levofloxacin intravenous infusion for anti-infection and phlegm-reduction, as well as other treatments, while the right thoracic cavity was intubated with a deep venous catheter for drainage of purulent pleural fluid and gas, as guided by CT imaging. More air bubbles overflowed from the right chest tubing when the patient coughed. The patient was diagnosed with BPFs. According to the complete CT image series, we found that the purulent cavity was located in the posterior and lower pleura cavity, a small channel between the bronchial stump of the posterior and outer basal segments of the lower lung and the purulent cavity was indistinctly visible, and the pulmonary infection focus was mainly located in the right lower lobe, so we inferred that the fistula was located at the bronchial stump of the posterior and outer basal segments of the right lower lung.
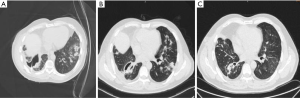
The patient received three deep venous drainage catheters in the 8th and 9th intercostal located scapular line to avoid the lung tissue under the CT guidance. The drainage flow was smooth, and the patient was given the cefoperazone sulbactam combined with levofloxacin for anti-infection treatment. The body temperature of the patient decreased to a normal range after several days, and the frequency of coughing was significantly reduced in comparison with the patient’s previous condition. In addition, we communicated with the patient about the necessity of tracheoscopy. However, he gave up the tracheoscopy due to the related complications. On the third day after catheter placement, the patient’s chest drainage tubes were found to be obstructed by pus, which looked granular. We originally wanted to improve drainage by careful flashing, and we flashed the tube under the right lateral lying position in order to reduce the spread of infection, but the amount of liquid and strength of flashing may not be well controlled. The patient’s cough and phlegm became worse on the same day, and the chest CT showed that the left lung infection was worse than that before the injection (Figure 2A), a finding considered to indicate that the chest cavity flushing infection could spread to the left lung. The patient was then given intensive assistance in body turn-over, back-patting, coughing up phlegm for position drainage and promotion of the discharge of infected substances in the left lung. The right chest drainage tube was re-set to insert a total of four tubes with a smooth flow to drain pus and gas. The patient’s cough and phlegm gradually improved. The patient achieved a normal temperature range. The amount of drainage fluid was gradually reduced to less than 30 mL/day. After 4 days, the chest CT was reviewed and indicated that the right chest abscess had shrunk (Figure 2B). Subsequently, the patient was set in the left lateral position for postural drainage, and the phlegm could be coughed up by the patient. After 9 days, the patient’s chest drainage substance comprised a small amount of light yellow liquid at approximately 10 mL/day. The chest CT showed that the right chest abscess was further reduced. The infection of the lung was clearly reduced. The patient was discharged from the hospital and was advised to continue to perform postural drainage on time every day to ensure timely discharge of phlegm. The patient undertook a regular outpatient review of chest CT to determine the residual cavity changes and lung infection reduction condition. Ten days after discharge from the hospital, chest CT showed that the right lung cavity was further reduced, the diameter was 3 cm, no gas-liquid level was seen, and the inflammation of the lung was significantly reduced. The chest drainage tube was removed. One month later, chest CT showed that the right lung cavity had disappeared, and the inflammation of the lung diminished (Figure 2C).
Discussion
Multidisciplinary discussion
Thoracic surgery discussion
Jun Wang (The People’s Hospital of Jiangsu Province): the symptoms of BPF, including cough and fever, appeared on the 4th day. The patient was considered as having an early stage fistula with regard to the intraoperative bronchial stump tissue damage. Although the early stage BPF could be repaired, surgery had a certain failure risk, owing to the heavy local tissue inflammation and edema, which would likely result in secondary BPF or pulmonary fistula. It was recommended that the patient first be given conservative drainage treatment. The drainage tube should be positioned under the guidance of CT. Pigtail tubing was the preferred choice of drainage tubing, because it would ensure drainage and reduce the discomfort of the patient. When the symptoms of the local residual cavity in the thoracic cavity and the general condition of the body stabilized, bronchoscopy could be performed to confirm the condition of the fistula. If the drainage tube is obstructed before the fistula is healed, open-window thoracostomy (OWT) is recommended to improve drainage. If the fistula still had not healed, surgery would be feasible. The surgery mode would be determined according to the inflammation of the fistula. If the inflammation of the fistula was well controlled, the cavity would be fully cleaned before repairing the fistula. If the local infection was severe, the adhesion would be fully separated, and the thoracic cavity would be cleaned before conducting the lobectomy. Latissimus dorsi or intercostal muscle flaps would be used to cover the bronchial stump in both cases.
Long Zhao (Zhejiang Xiaoshan Hospital): replacing the larger diameter closed thoracic drainage tube for full drainage to less than 50 mL/day was recommended, and extubation should not be considered until the drainage fluid becomes clear. Meanwhile, the usage of antibiotics should be adjusted according to the results of drug sensitivity. Alternatively, cefoperazone sodium tazobactam or linezolid intravenous infusion could be used first. When the body temperature is well controlled, cefoperazone sodium tazobactam combined with levofloxacin intravenous infusion could be used. The anti-infective treatment was recommended for more than four weeks. In addition, consumptive infections require adequate nutritional support, which is required for this patient. Generally, small lesions of BPF can heal by themselves after good infection control, leaving only the loculated residual cavity, which can be continuously drained completely to form a sinus or subjected to surgical debridement. If conservative drainage and anti-infection treatment are ineffective, fiber optic bronchoscopy could be used to examine or block the fistula. However, stent blockage has a risk of expanding the small fistula. If all the above strategies were ineffective, the last approach would be surgical treatment. On the one hand, the chest cavity would need to be thoroughly washed, because the entrapment effusion is often difficult to drain completely; on the other hand, the specific condition of the fistula could be explored. Surgical treatment could at least accurately place the drainage tube around the fistula to facilitate post-operative drainage, and the fistula could be sutured together if possible. Furthermore, after the patient has a segmentectomy, the worst option would be to have a lobectomy to further dissect the fistula.
Discussion from respiratory medicine departments
Zengli Zhang (The Second Affiliated Hospital of Soochow University): the patient had a lower lobe bronchial stump fistula, and the abscess was located in the posterior and lower thoracic cavity. If the drainage was unobstructed in conjunction with the effective antibiotic treatment, the symptoms of systemic and local infections could be quickly relieved. Therefore, conservative treatment was recommended. It was necessary to improve the drainage early to accelerate the discharge of pus in the abscess. Fiber optic bronchoscopy should be used to determine the size of the fistula. If the diameter of the fistula is less than 5 mm, use of a gel could be considered to seal the fistula. If the diameter is greater than 5 mm, the sealing effect would not be good. It was recommended to continue to improve the chest drainage. The chest tube should be re-set or replaced when necessary. Flushing the abscess was not recommended, to avoid the spread of intrapulmonary infection. The use of sensitive antibiotics for systemic therapy requires antibiotics active against Gram-negative bacteria to enhance the anti-infective treatment.
Discussion from intervention departments
Long Chen (The Second Affiliated Hospital of Soochow University): the patient had an abscess cavity formed in the thoracic cavity with acute infection. It was recommended to place the drainage tube under the guidance of CT. It would be difficult to place the chest tube by the chest wall, because it would be likely to damage the lung tissue and introduce a risk of bleeding. Moreover, the abscess was close to the scapula. The drainage tube placed through the lateral chest wall would be next to the scapula and could easily cause the drainage tube to escape as a result of upper limb movement. The closest distance between the abscess and the chest wall was located between the 8th and 9th ribs of the lower scapula. This treatment strategy is highly safe, and a pigtail drainage tube could be used, which would ensure smooth flow and reduce the patient’s discomfort. The pus was viscous. If the drainage condition is poor because of the fine drainage tube, a catheter could be punctured again under CT guidance. Alternatively, the pus could be carefully flushed through the drainage tube to improve the drainage flow. From a safety viewpoint, it was recommended that a small amount of saline be first used to flush the cavity. Flushing with a syringe is convenient to control the washing speed. During the process, the operator should pay attention to the amount of flushing fluid, evaluate whether the flow is smooth, and avoid airway infection. In reviewing the CT, if there were signs of infection and dissemination, it was recommended to stop flushing.
Discussion from department of rehabilitation
Liang Zhou (People’s Hospital of Zhejiang Province): the patient had a residual abscess in the thoracic cavity with intrapulmonary dissemination, but the abscess is small. It was recommended to drain the abscess first and insert a #32 chest tube to allow complete drainage. After intubation, it could be kept under low vacuum pressure to maintain smooth drainage. Meanwhile, the thoracic cavity could be flushed with physiological saline. When the symptoms of systemic infection including cough and fever improve and the patient can effectively cough up the sputum, it was recommended to perform postural drainage from the affected side of the patient with a gradual transition to the healthy lateral position. The cough should be strengthened to facilitate sputum drainage and concurrently help promote lung recruitment, eliminate the residual cavity, and promote respiratory function recovery.
Summary of discussion
BPF is defined as an abnormal channel formed between the bronchus and the pleura at all levels. It is one of the most severe complications after lung surgery including pneumonectomy, lobectomy, and segmentectomy. BPF is dangerous and complicated. It can easily cause severe infections and even septic shock (1). The incidence rate of BPF is between 1.5% and 15%, and the mortality rate is 25% to 71% (1-4). It is still difficult to treat BPF clinically. Non-surgical treatments include chest drainage and bronchoscopy, and surgical treatments include fistula flushing repair and enlarged lung resection to close the fistula (4).
Regarding the follow-up treatment to this patient, there are slight differences between the principles of surgical and non-surgical treatment. The advantage of surgical treatment is that it can immediately close the fistula and shorten the recovery period. Duan Liang and Chen Xiaofeng have performed early surgical treatment on 25 patients with a BPF fistula of 3–6 mm. The average hospital stay was 33 days (5). However, surgical treatment is more invasive. In addition, the possible chest infection after surgery, recurrence of BPF, and severe systematic infection may cause surgical failure. Uramoto and Hanagiri have summarized the treatment experience of 19 BPF cases encountered in 31 years, 11 of which failed to repair and resulted in death with a failure rate of 57.9% (6). The advantages of conservative drainage therapy include avoiding reoperation and related complications. In comparison with patients undergoing surgical treatment, patients receiving conservative treatment can usually afford the hospitalization costs of antibiotics and chest drainage flushing, and can avoid the extra surgery costs and related costs of consumables. Fuso has compared conservative drainage therapy with surgical treatment of BPF and found no significant difference in healing time between the two strategies (7). For the treatment of this case, most thoracic surgeons and respiratory doctors recommend the conservative chest drainage treatment, which is considered to have a high success rate for localized BPF in the lower respiratory segment of the lung.
In conservative chest drainage treatment, the consensus opinion is that drainage should be maintained to the maximum extent. In terms of drainage tube selection and placement, there are no standard guidelines in clinical practice, and we believe that procedures are mainly determined according to the position of the abscess and the conditions for unobstructed abscess drainage.
If pus obstructs the drainage tube during the drainage process, the consensus of physicians is that drainage should be improved immediately through various procedures. Interventional surgeons and some thoracic surgeons believe that the drainage tube should be repositioned or flushed with saline to facilitate drainage, some thoracic surgeons recommend thoracic OWT to improve drainage. Antonio Mazzella has used OWT on 27 patients with BPF for whom conservative drainage therapy was ineffective. The chest infections of those patients were effectively controlled (4), but OWT required fenestration, and the chest wall window required additional repair, thus resulting in high trauma and a low patient acceptance rate. Massera et al. have shown that OWT has good effects on patients with long-term indwelling drainage tubes who cannot control their chest infections. However, an average of 8.5 months is necessary post-surgery to close the chest open window, thus leading to slow recovery in patients after surgery (8).
Some thoracic surgeons recommend flushing the residual cavity after the closing the fistula to promote healing of the residual cavity. In addition, in auxiliary treatment after catheter drainage, the respiratory physicians recommend that the patient maintain the affected lateral position to prevent early infection and spread when large amounts of drainage fluid are present. When the patient’s general condition is stable, the drainage can be reduced, and the patient can gradually change position with intermittent coughing and phlegm drainage. Rui et al. found that when the patient’s chest drainage was less than 30 mL/day, and the patient’s cough was effective, the drainage position was safe and effective and promoted healing in 13 post-operative BPF patients (9).
In the conservative treatment process, most physicians recommend that bronchoscopy be performed to determine the healing of the fistula and to decide the next treatment plan after the patient’s condition is stable. If the fistula is not closed, the physicians from the respiratory department and some thoracic surgeons believe that when the fistula is less than 3–5 mm, the endoscopic sealing method should be attempted. Studies have shown that when the fistula is less than 3 mm, the orally injected gel sealing method had a higher success rate (10). A larger failure rate occurs when the fistula is larger, and the sealing gel is likely to be coughed out or spill into the remaining bronchus. Cardillo et al. have reported that BPF patients receiving endoscopic injection had a failure rate of 34.3% (11). Therefore, some thoracic surgeons recommend thoracotomy to remove the right lower lobe or to repair the stump if the patient’s fistula does not heal.
For the treatment of this patient, after multidisciplinary discussion, it was decided to follow a conservative treatment approach. A soft drainage tube was placed at the lowest position of the abscess under CT guidance. When the drainage had no obstacles and the abscess was reduced, assisting postural drainage was gradually used to facilitate drainage.
Conclusions
The current multidisciplinary treatment model has been widely used worldwide. This treatment model integrates the opinions of experts from various disciplines to provide one-stop services for patients and helps to provide more comprehensive and reasonable diagnosis and treatment decisions for critically ill patients (12). Because of its rapid development, BPF can easily cause respiratory failure in patients. Early diagnosis of BPF can minimize patient mortality. According to reports in the literature, the average extubation time of patients is 40.5 days with conservative treatment of BPF, whereas the average extubation time of patients is 33 days with surgical treatment of BPF (5,9). The patient underwent CT-guided thoracic catheter drainage treatment, as advised by a multidisciplinary team. The best therapy plan was chosen through the analysis of thoracic surgery and respiratory department, also the most accurate operation and exercise method was selected by the experts OS Intervention department and Rehabilitation department. Later, the patient underwent assisted postural drainage in combination with anti-infection treatment and nutritional support treatment. The patient recovered well. The patient’s symptoms were completely relieved. The bronchial stump fistula healed, and the chest abscess disappeared. A total of 26 days elapsed between the occurrence of BPF and the extubation of the drainage tube. The patient had good compliance during the process, and the treatment results were satisfactory. Therefore, the conservative treatment strategy was effective for this patient. The reasons for the success may include the following: the position of the fistula was low; the local abscess had lung tissue coverage, and thus could easily be localized and was not likely to cause extensive fistula; there were no solid traits in the abscess; the puncture and drainage were smooth; the treatment trauma was low; and the patient had good compliance and cooperated with postural drainage.
Acknowledgments
Funding:
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-1930
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-1930). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oizumi H, Kato H, Endoh M, et al. Management of Bronchial Stumps in Anatomic Lung Segmentectomy. Ann Thorac Surg 2016;101:2120-4. [Crossref] [PubMed]
- Birdas TJ, Morad MH, Okereke IC, et al. Risk factors for bronchopleural fistula after right pneumonectomy: does eliminating the stump diverticulum provide protection? Ann Surg Oncol 2012;19:1336-42. [Crossref] [PubMed]
- Li Y, Luan Y, Cui YB, et al. Management of Early Bronchopleural Fistula After Pneumonectomy. Zhonghua Yi Xue Za Zhi 2016;96:1692-5. [PubMed]
- Mazzella A, Pardolesi A, Maisonneuve P, et al. Bronchopleural Fistula After Pneumonectomy: Risk Factors and Management, Focusing on Open-Window Thoracostomy. Semin Thorac Cardiovasc Surg 2018;30:104-13. [Crossref] [PubMed]
- Duan L, Chen X, Zhu Y, et al. Repair of thoracotomy fistula for early bronchopleural fistula after pneumonectomy. Chinese Journal of Thoracic Cardiovascular Surgery 2012;28:362-4.
- Uramoto H, Hanagiri T. The development of Bronchopleural Fistula in lung cancer patients after major surgery: 31 years of experience with 19 cases. Anticancer Res 2011;31:619-24. [PubMed]
- Fuso L, Varone F, Nachira D, et al. Incidence and management of post-lobectomy and pneumonectomy Bronchopleural Fistula. Lung 2016;194:299-305. [Crossref] [PubMed]
- Hysi I, Rousse N, Claret A, et al. Open window thoracostomy and thoracoplasty to manage 90 postpneumonectomy empyemas. Ann Thorac Surg 2011;92:1833-9. [Crossref] [PubMed]
- Mao R, Ying PQ, Xie D, et al. Conservative management of empyema-complicated post-lobectomy bronchopleural fistulas: experience of consecutive 13 cases in 9 years. J Thorac Dis 2016;8:1577-86. [Crossref] [PubMed]
- Salik I, Abramowicz AE. Bronchopleural Fistula. Treasure Island (FL): StatPearls Publishing, 2020.
- Cardillo G, Carbone L, Carleo F, et al. The Rationale for Treatment of Postresectional Bronchopleural Fistula: Analysis of 52 Patients. Ann Thorac Surg 2015;100:251-7. [Crossref] [PubMed]
- Munshi A, Sengar M. Multidisciplinary care in oncology: Are we united? Acta Oncol 2011;50:314-6. [Crossref] [PubMed]


