Reconsidering the therapeutic use for vacuum-assisted breast biopsy in breast cancer patients: a retrospective single-center study
Introduction
Based on the latest data in 2019 (1), breast cancer accounts for approximately 30% of new cancer cases and is the most prevalent malignant tumor in American women. New techniques in medical imaging, which include mammography, ultrasonography (US), and magnetic resonance imaging (MRI), have improved the early diagnosis of breast cancer. Furthermore, these imaging modalities have facilitated imaging-guided breast biopsy, including image-guided fine needle aspiration biopsy (FNAB), core needle biopsy (CNB), vacuum-assisted breast biopsy (VABB), and fine needle localization. Moreover, the concept of minimally invasive surgery has been increasingly applied to breast cancer treatment.
The VABB technique has been widely used for diagnosing breast lesions (2), especially for multiple or non-palpable breast masses and microcalcifications of extreme size (3). In 2002, the US Food and Drug Administration (FDA) approved VABB for the removal of benign lesions, as it is considered safe, provides effective complete excision, and cosmetic benefits (4). Previous studies (3,5) have evaluated the usefulness and safety of VABB; it is believed that VABB could replace FNAB and CNB for the diagnosis of breast disease. In a recent systematic review and meta-analysis of 20,000 people from 36 longitudinal studies (6), the pooled data suggested that VABB with US or mammography could be promising for the diagnosis of breast disease.
The diagnostic usefulness of VABB in breast diseases has been proved over the years (7,8), and is even being explored in breast lesions that cannot be detected by other imaging modalities (9,10). The development of VABB has enabled the excision of benign breast tumors and complete excision is possible without residual tumor tissue (3,6). VABB has proven useful even for phyllodes tumors, which have a tendency of recurrence; using VABB to excise benign phyllodes tumors showed a low recurrence rate of 7.46% during the follow-up period (11). Researchers have also tried to evaluate the use of VABB in breast-conserving surgery (12,13). However, there is little evidence regarding the therapeutic indications for VABB in breast cancer, given the lack of long-term follow-up data.
Therefore, this single-center retrospective study evaluated Chinese breast cancer patients who had undergone VABB. We aimed to evaluate the rate of residual tumor remaining after the VABB procedure, the time to surgery after VABB, and any differences in pathological findings between the excised and residual tumors. We presented the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2906).
Methods
This study’s retrospective protocol was approved by the Peking Union Medical College Hospital Institutional Review Board (S-K930). Following consensus guidelines (8), we confirmed that there were no contraindications to surgery. Only mass lesions that could be positioned by preoperative US-guided VABB were included. A Samsung Medison linear transducer was utilized for the US examination. The Mammotome systems were from Johnson Companies and appropriate types of rotary cutter were selected according to the size of the targeted lesions. The VABB process included routine disinfection, VABB equipment preparation, anesthesia, placement of the rotary cutter and performance of the rotary excision until the operation was completed under US monitoring. At the end of the resection operation, re-examination of the tumor bed under US was also performed to ensure that there was no residual lesion.
We identified 89 Chinese women who underwent VABB between January 2011 and December 2018 and were confirmed as having breast cancer, including in situ or invasive malignant lesions. The pathological results of all enrolled patients from the VABB were evaluated by at least two pathologists at our hospital. The pathological diagnostic criteria were based on the World Health Organization Classification of Breast Tumors (14), and molecular classification was based on the St. Gallen Consensus criteria (15). Data that couldn't be classified were recorded as “unknown” in the tables. Patients were excluded if they were pregnant or lactating, had distant metastasis at the initial diagnosis, or had undergone palliative resection or neoadjuvant chemotherapy.
Two ultrasonologists assessed the breast lesion’s morphology and blood flow signals. Each patient was provided a personalized surgical plan based on their preference and the National Comprehensive Cancer Network guidelines (16). Postoperative adjuvant chemotherapy, radiotherapy, endocrine therapy, or targeted therapy was provided according to the pathological results from the resected and residual tumors. Targeted therapy mainly referred to adjuvant trastuzumab in the treatment of human epidermal growth factor receptor 2 (Her-2) positive breast cancer patients.
The enrolled patients were divided into two groups: residual and non-residual. Patients in the residual group had a residual tumor in the postoperative pathology of open surgical excision, whereas no residual tumor was identified in the non-residual group. All patients underwent follow-up using clinical examinations and comprehensive imaging examinations, including US, mammography, computed tomography, and even positron emission tomography if necessary. Because death was a rare outcome in this time frame and it was too short to analyze overall survival, we used disease-free survival (DFS) as the endpoint, defined as the duration from the date of initial diagnosis of breast cancer to the first time of breast cancer-specific recurrence or distant metastasis. Recurrence or distant metastasis during the follow-up period was evaluated using the Kaplan-Meier method. All statistical analyses were performed using IBM SPSS software (version 24.0), with the χ2 test or Fisher’s exact probability text as appropriate. Differences were considered statistically significant at P values of <0.05.
Results
Patient characteristics
Based on the inclusion and exclusion criteria, 89 patients were included, with a mean original lesion size of 1.76±0.90 cm. The mean number of biopsy samples was 8.9±6.6 in our study, since most of the tumors were less than 2 cm in size. The number of samples varied due to the size and shape of different lesions; precise positioning is also an important factor when considering the number of specimens removed. Among the patients, 62 had residual tumors after the VABB (69.6%). The mean patient age was 44.3 years (range: 28–57 years), although no significant inter-group differences were observed in age (Table 1). When we compared the groups with and without residual tumor, we failed to detect significant differences in the maximum resected tumor size (1.30±0.42 vs. 1.22±0.39 cm, P=0.418), Breast Imaging-Reporting and Data System (BI-RADS) classification (17), histological type, and histological grade. The difference in the breast cancer subtypes may have been related to the unclear immunohistochemical staining results for some patients.
Table 1
| Variable | No. (%) | χ2 | P | |
|---|---|---|---|---|
| Residual group | Non-residual group | |||
| Total | 62 | 27 | ||
| Age group (years) | 0.059 | 0.808 | ||
| ≤35 | 8 (12.9%) | 4 (14.8%) | ||
| >35 | 54 (87.1%) | 23 (85.2%) | ||
| BI-RADS classification | 1.220 | 0.921 | ||
| 3 | 27 (43.5%) | 13 (48.1%) | ||
| 4 | 29 (46.8%) | 13 (48.1%) | ||
| 5 | 4 (6.5%) | 1 (3.7%) | ||
| Unknown | 2 (3.2%) | 0 (0.0%) | ||
| Maximum size of resected tumors | 0.816 | 0.418 | ||
| Mean ± SD (cm) | 1.30±0.42 | 1.22±0.39 | ||
| Histological type | 0.836 | 0.658 | ||
| DCIS | 11 (17.7%) | 7 (25.9%) | ||
| IDC | 29 (46.8%) | 12 (44.4%) | ||
| DCIS + IDC | 22 (35.5%) | 8 (29.6%) | ||
| Histological grade | 3.774 | 0.169 | ||
| I | 19 (30.6%) | 10 (37.0%) | ||
| II | 28 (45.2%) | 15 (55.6%) | ||
| III | 11 (17.7%) | 2 (7.4%) | ||
| Unknown | 4 (6.5%) | 0 (0.0%) | ||
| Breast subtype | 11.324 | 0.010 | ||
| Luminal A | 24 (38.7%) | 17 (63.0%) | ||
| Luminal B | 28 (45.2%) | 4 (18.5%) | ||
| Her-2 | 0 (0.0%) | 0 (0.0%) | ||
| Triple negative | 4 (6.5%) | 3 (18.5%) | ||
| Unknown | 6 (9.7%) | 0 (0.0%) | ||
| Lesion size by US after VABB | 3.117 | 0.002 | ||
| Mean ± SD (cm) | 1.76±0.49 | 1.36±0.68 | ||
| Lesion morphology by US after VABB | 38.149 | 0.000 | ||
| Regular morphology | 0 (0.0%) | 14 (51.9%) | ||
| Irregular morphology | 62 (100.0%) | 13 (48.1%) | ||
| Blood flow signal grade via US after VABB | 18.773 | 0.000 | ||
| 0 | 25 (40.3%) | 21 (77.8%) | ||
| I | 8 (12.9%) | 6 (22.2%) | ||
| II–III | 29 (46.8%) | 0 (0.0%) | ||
SD, standard deviation; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; Her-2, human epidermal growth factor receptor 2; US, ultrasound; VABB, vacuum-assisted breast biopsy.
All patients underwent breast US before surgery, which revealed a significant inter-group difference in the tumor bed lesions’ maximum diameters (1.76±0.49 vs. 1.36±0.68 cm, P=0.002). The US-determined morphology and blood flow signals were also evaluated preoperatively according to the BI-RADS system. All the patients in the residual group had irregularly shaped lesions, while 52.6% of the patients in the non-residual group had irregular morphology. The blood flow signal grades were predominantly grade 0–I in the non-residual group, while approximately one-half of the results were grade II–III in the residual group (P=0.000).
Comparison of treatments and TNM staging between the two groups
The mean time from VABB to surgery was 29.97±11.73 days in all patients. Table 2 showed that both groups had similar time to surgery after the VABB (31.45±11.61 vs. 26.56±11.50 days, P=0.072). There were also no significant differences in surgical technique, chemotherapy, endocrine therapy, radiotherapy, targeted therapy, or breast-conservation rate (40.3% vs. 44.4%).
Table 2
| Variable | No. (%) | χ2 | P | |
|---|---|---|---|---|
| Residual group | Non-residual group | |||
| Time to surgery after VABB | 1.841 | 0.072 | ||
| Mean ± SD (days) | 31.45±11.61 | 26.56±11.50 | ||
| Surgery type | 0.132 | 0.717 | ||
| BCS | 25 (40.3%) | 12 (44.4%) | ||
| Mastectomy | 37 (59.7%) | 15 (55.6%) | ||
| Axillary staging methods | 3.828 | 0.147 | ||
| SLNB | 26 (41.9%) | 9 (33.3%) | ||
| ALND | 36 (58.1%) | 18 (66.7%) | ||
| Hormone therapy | 3.013 | 0.083 | ||
| Yes | 58 (93.5%) | 22 (81.5%) | ||
| No | 4 (6.5%) | 5 (18.5%) | ||
| Chemotherapy | 3.174 | 0.075 | ||
| Yes | 26 (41.9%) | 6 (22.2%) | ||
| No | 36 (58.1%) | 21 (77.8%) | ||
| Radiotherapy | ||||
| Yes | 25 (40.3%) | 8 (29.6%) | 0.922 | 0.337 |
| No | 37 (59.7%) | 19 (70.4%) | ||
| Targeted therapy | ||||
| Yes | 7 (11.3%) | 1 (3.7%) | 1.323 | 0.250 |
| No | 55 (88.7%) | 26 (96.3%) | ||
| Size of residual tumors | 4.466 | 0.000 | ||
| Mean ± SD (cm) | 0.69±0.81 | 0±0 | ||
| Tumor staging | 19.637 | 0.001 | ||
| Tis | 4 (6.5%) | 0 (0.0%) | ||
| T1a | 0 (0.0%) | 0 (0.0%) | ||
| T1b | 2 (3.2%) | 7 (25.9%) | ||
| T1c | 38 (61.3%) | 20 (74.1%) | ||
| T2 | 17 (27.4%) | 0 (0.0%) | ||
| T3 | 1 (1.6%) | 0 (0.0%) | ||
| TNM staging | 16.879 | 0.054 | ||
| 0 | 7 (11.3%) | 0 (0.0%) | ||
| I | 35 (56.5%) | 27 (100.0%) | ||
| II | 12 (19.4%) | 0 (0.0%) | ||
| III | 8 (12.9%) | 0 (0.0%) | ||
BCS, breast-conserving surgery; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; Tis, carcinoma in situ; T1a, tumor size of 0.1–0.5 cm; T1b, tumor size of 0.5–1 cm; T1c, tumor size of 1–20 mm; T2, tumor size of 20–50 mm; T3, tumor size of >50 mm.
The average maximum diameter of the residual tumors was 0.69 cm (range: 0.1–6 cm). All patients were staged according to the 8th edition of the American Joint Committee on Cancer guidelines (18). The primary tumors in the non-residual group were all <2 cm, while 29% of patients in the residual group had T classifications of T2–3, and this difference was statistically significant (P=0.001). All patients in the non-residual group were considered TNM stage I, while 32.3% of the patients in the residual group were considered stage II–III (P=0.054). We also made subgroup analyses to see how tumor size could influence the rate of total resection of the lesions (Table 3). There was a rising residual rate with an increasing tumor size.
Table 3
| Variable | No. (%) | P | |
|---|---|---|---|
| Residual group | Non-residual group | ||
| Tumor size T (cm) | <0.001 | ||
| T ≤1 | 2 (22.2%) | 7 (77.8%) | |
| 1< T ≤2 | 38 (65.5%) | 20 (34.5%) | |
| T >2 | 17 (100%) | 0 (0%) | |
Pathological consistency between the resected and residual tumors
Some researchers have expressed concerns regarding the accuracy of preoperative VABB for determining the pathological characteristics of breast diseases (19,20). Therefore, we compared the pathological findings between the excised and residual tumors (Table 4). Twenty-nine patients were diagnosed with invasive ductal carcinoma based on the VABB, which was ultimately confirmed by the postoperative pathological diagnosis. In the residual group, comparisons of the histopathological findings between the resected and residual tumors revealed no significant differences (all P>0.05).
Table 4
| Variable | No. (%) | χ2 | P | |
|---|---|---|---|---|
| Resected tumors | Residual tumors | |||
| Histological type | 1.567 | 0.457 | ||
| DCIS | 11 (17.7%) | 16 (25.8%) | ||
| IDC | 29 (46.8%) | 29 (46.8%) | ||
| DCIS + IDC | 22 (35.5%) | 17 (27.4%) | ||
| Histological grade | 7.477 | 0.058 | ||
| I | 19 (30.6%) | 15 (24.2%) | ||
| II | 28 (45.2%) | 40 (64.5%) | ||
| III | 11 (17.7%) | 7 (11.3%) | ||
| Unknown | 4 (6.5%) | 0 (0.0%) | ||
| Breast subtype | 10.412 | 0.015 | ||
| Luminal A | 24 (38.7%) | 14 (22.6%) | ||
| Luminal B | 28 (45.2%) | 34 (54.8%) | ||
| Her-2 | 0 (0.0%) | 0 (0.0%) | ||
| Triple negative | 4 (6.5%) | 0 (0.0%) | ||
| Unknown | 6 (9.7%) | 14 (22.6%) | ||
IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; Her-2, human epidermal growth factor receptor 2.
Follow-up
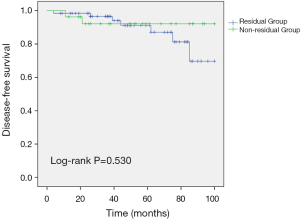
All patients had available follow-up data, with a median follow-up time of 52.3 months (range: 8–100 months). Patients were censored on September 30, 2019, at which point all patients in the non-residual group were alive, including 2 patients who had experienced local recurrence. At that same point, some patients in the residual group had experienced local recurrence of breast cancer (3 patients, 4.8%), bone metastasis (1 patient, 1.6%), or lung metastasis (3 patients, 4.8%). When we compared the residual and non-residual groups, we failed to detect significant differences in DFS (log-rank P=0.53) (Figure 1).
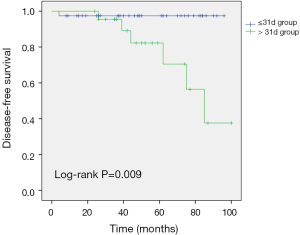
We also subdivided the residual group according to the time between the VABB and surgery (cut-off: 31 days), and our analyses failed to detect significant differences in tumor classification (P=0.73) or TNM stage (P=0.621). A longer time to surgery was associated with significantly shorter DFS in the residual group (log-rank P=0.009) (Figure 2).
Discussion
The VABB device was developed in the 1990s by California radiologist Fred Burbank and medical device engineer Mark Retchard to address the limitations of CNB (21). In 2002, VABB was approved by the US FDA as a diagnostic tool for localized biopsy of breast lesions. The VABB device consists of a rotating cutter head and a vacuum suction system, which provides clear benefits compared with CNB. For example, tissues can be sucked out through the vacuum system, which allows for multiple tissue samples to be obtained without repeated punctures (22). The VABB was originally intended to facilitate a pathological diagnosis, although VABB has been clinically used for complete excision of benign breast tumors (23), especially unilateral or bilateral multiple benign breast lesions, clinically non-palpable breast lesions, or microcalcifications identified using mammography (24,25).
However, the therapeutic value of VABB for breast cancer remains controversial, given the lack of high-quality data, and VABB is not currently recommended for breast cancer excision (3). A retrospective study (26) of 5,232 patients undergoing VABB revealed 61 malignant lesions (44 lesions were carcinoma in situ and 17 lesions were invasive carcinomas), and the study showed that VABB provided 100% sensitivity for breast cancer detection. We conclude that VABB could be used for the detection of early breast cancer and as a clinical diagnostic technique. Given that malignant tumors could invade and spread to other parts of the body, they are more likely to be incompletely resected, especially for irregularly shaped tumors that are identified via US before VABB (27). Previous studies (12,28,29) have also suggested that the residual tumor rates for breast cancer were 45.5–67%, which is similar to our rate of 69.6%. Nevertheless, it has been emphasized that maximal tumor control via breast-conserving surgery should be a priority (15), and it is not advisable to avoid radical resection because of the minimal invasiveness or cosmetic benefits of VABB.
The present study revealed that US-based morphology and blood flow signals from before surgery were related to residual tumor. This may be because invasive breast cancer cells can exhibit “burr” or “crab foot” US signs, with rich blood flow around the tumor. Some authors (30) suggests that single tumors with good morphology and benign tendency should be resected first, although the same head should not be used to remove bilateral or multiple lesions, given the possibility of unsuspected breast cancer. Thus US-determined diameter and morphology of tumor bed after VABB may have an indication for surgeons to decide the resection range in open surgery. In addition, the present study revealed inter-group differences in residual tumor diameter and T classification. Since tumor size is an important factor for minimally invasive treatment (31), we have also seen a rising residual rate with an increasing tumor size in this study. This is important because larger tumors (>3 cm) may not be feasibly removed via VABB, given that the rotating cutter head is approximately 2.6 cm (32). If negative margins cannot be confirmed, open surgery should be considered. Some researchers (13) have also tried to combine VABB with endoscopic minimally invasive breast-conserving surgery to improve the cosmetic outcomes, although they acknowledged that >2 cm tumors are not suitable for this technique, which also requires a longer procedure than open surgery.
The follow-up data from this study revealed that the residual tumor group had more recurrences and metastases, which may be related to the ratio of stage II–III patients. Nevertheless, we did not detect significant differences in DFS, given that most patients had early breast cancer (TNM stage 0–I). Thus, we evaluated the relevance of the time from VABB to surgery, and found that longer times were associated with shorter DFS in the residual group. Relative to open surgery under direct vision, the main complications of VABB are pain and ongoing post-procedural bleeding (3). Furthermore, we speculate that there would be more circulating tumor cells in patients with a long time between VABB and surgery. Liquid biopsy refers to the use of circulating tumor DNA, circulating tumor cells and other non-invasive biomarkers such as long-stranded non-coding RNA, messenger RNA and microRNA, proteins and exons for early diagnosis, prognosis, monitoring clinical progression and treatment response in patients (33). Recent studies have applied liquid biopsy to traditional breast cancer screening for personalized diagnosis and breast cancer management (34); this may be a potentially useful application for identifying breast cancer patients with residual tumors after VABB.
Moreover, VABB is now mainly conducted in the outpatient department in China. Cross-provincial medical treatments could be rather complicated in some cases due to different medical care payment systems. Many patients will have to pay for VABB in the outpatient department at their own expense. We believe that, further selection of proper breast lesions for VABB would be a valid option in order to reduce the excessive operative costs (35,36).
Our study included breast cancer patients with survival data, and we also found an association between the time from VABB to open surgery with DFS in the residual group, which has seldom been provided in previous studies. However, the present study has several limitations. First, it was a retrospective review of a small sample of breast cancer patients who were treated at a single center. Given the small sample size, additional studies are needed to explore potential differences in the molecular subtypes. Second, not all the patients were eligible for or would consent to VABB. Third, the median follow-up time was only 52.3 months and longer follow-up may be needed to detect recurrence and metastasis from early breast cancer. Fourth, because of technical limitations at our center, the subtyping of the cases was imprecise and could not be assigned for approximately 25% of patients. Further studies may be needed to clarify any differences regarding breast cancer subtype.
Conclusions
The present study revealed that VABB was associated with a substantial residual tumor rate in breast cancer cases, and that further extended surgery is always essential. Based on our experience, VABB should not be considered for malignant breast tumors with a diameter of >2 cm or in cases with an anticipated prolonged time to surgery. Nevertheless, given the limited existing data, additional large studies with long follow-ups are needed to clarify the safety and efficacy of VABB for breast cancer. Moreover, it is important to include cost analyses based on the overall economic cost of open surgery.
Acknowledgments
We are extremely grateful for our colleagues for their support in collection of follow-up data during the whole study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2906
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2906
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-19-2906
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2906). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Peking Union Medical College Hospital Institutional Review Board (No. S-K930). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Yu YH, Liang C, Yuan XZ. Diagnostic value of vacuum-assisted breast biopsy for breast carcinoma: a meta-analysis and systematic review. Breast Cancer Res Treat 2010;120:469-79. [Crossref] [PubMed]
- Bennett IC, Saboo A. The evolving role of vacuum assisted biopsy of the breast: A progression from fine-needle aspiration biopsy. World J Surg 2019;43:1054-61. [Crossref] [PubMed]
- Hahn M, Krainick-Strobel U, Toellner T, et al. Interdisciplinary consensus recommendations for the use of vacuum-assisted breast biopsy under sonographic guidance: First update 2012. Ultraschall Med 2012;33:366-71. [Crossref] [PubMed]
- Park HL, Kim KY, Park JS, et al. Clinicopathological analysis of ultrasound-guided vacuum-assisted breast biopsy for the diagnosis and treatment of breast disease. Anticancer Res 2018;38:2455-62. [PubMed]
- Fang M, Liu G, Luo G, et al. Feasibility and safety of image-guided vacuum-assisted breast biopsy: A PRISMA-compliant systematic review and meta-analysis of 20, 000 population from 36 longitudinal studies. Int Wound J 2019;16:1506-12. [Crossref] [PubMed]
- Park HL, Hong J. Vacuum-assisted breast biopsy for breast cancer. Gland Surg 2014;3:120-7. [PubMed]
- Fan Z, Wang J, Hua B, et al. Expert consensus and surgery guideline for ultrasound guided vacuum-assisted breast biopsy (2017 edition). Chinese J Pract Surg 2017;37:1374-6.
- Okamoto S, Chen ST, Covelli JD, et al. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: imaging characteristics, outcome of surgical excision or imaging follow-up. Breast Cancer 2019; [Crossref] [PubMed]
- Penco S, Rotili A, Pesapane F, et al. MRI-guided vacuum-assisted breast biopsy: experience of a single tertiary referral cancer centre and prospects for the future. Med Oncol 2020;37:36. [Crossref] [PubMed]
- Park HL, Pyo YC, Kim KY, et al. Recurrence rates and characteristics of phyllodes tumors diagnosed by ultrasound-guided vacuum-assisted breast biopsy (VABB). Anticancer Res 2018;38:5481-7. [Crossref] [PubMed]
- Cangiarella J, Gross J, Symmans WF, et al. The incidence of positive margins with breast conserving therapy following mammotome biopsy for microcalcification. J Surg Oncol 2000;74:263-6. [Crossref] [PubMed]
- Xu Y, Ming J, Zhou Y, et al. Mammotome-assisted endoscopic breast-conserving surgery: a novel technique for early-stage breast cancer. World J Surg Oncol 2014;12:99. [Crossref] [PubMed]
- Sinn HP, Kreipe H. A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel) 2013;8:149-54.
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2017;28:1700-12. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Abraham J, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:452-78. [Crossref] [PubMed]
- Rao AA, Feneis J, Lalonde C, et al. A pictorial review of changes in the BI-RADS fifth edition. Radiographics 2016;36:623-39.
- Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol 2018;25:1783-5.
- Fujita T, Sawaki M, Hattori M, et al. The accuracy of preoperative ultrasonography guided vacuum-assisted breast biopsy in determining histological type, ER Status, PgR Status, HER2 Status and Ki67 Level in invasive breast cancer. Cancer Res 2011;71:P5-11-01.
- Underestimation of breast cancer in intraductal papillomas treated with vacuum-assisted core needle biopsy. Ginekol Pol 2019;90:122-7. [Crossref] [PubMed]
- Burbank F. Stereotactic breast biopsy of atypical ductal hyperplasia and ductal carcinoma in situ lesions: improved accuracy with directional, vacuum-assisted biopsy. Radiology 1997;202:843-7. [Crossref] [PubMed]
- Lee SH, Kim EK, Kim MJ, et al. Vacuum-assisted breast biopsy under ultrasonographic guidance: analysis of a 10-year experience. Ultrasonography 2014;33:259-66. [Crossref] [PubMed]
- Ding B, Chen D, Li X, et al. Meta analysis of efficacy and safety between Mammotome vacuum-assisted breast biopsy and open excision for benign breast tumor. Gland Surg 2013;2:69-79. [PubMed]
- Mariscotti G, Durando M, Robella M, et al. Mammotome(®) and EnCor (®): comparison of two systems for stereotactic vacuum-assisted core biopsy in the characterisation of suspicious mammographic microcalcifications alone. Radiol Med 2015;120:369-76. [Crossref] [PubMed]
- Liu J, Huang L. Image-guided vacuum-assisted breast biopsy in the diagnosis of breast microcalcifications. J Int Med Res 2018;46:2743-53. [Crossref] [PubMed]
- Pan S, Liu W, Jin K, et al. Ultrasound-guided vacuum-assisted breast biopsy using Mammotome biopsy system for detection of breast cancer: results from two high volume hospitals. Int J Clin Exp Med 2014;7:239-46. [PubMed]
- Meroni S, Bozzini AC, Pruneri G, et al. Underestimation rate of lobular intraepithelial neoplasia in vacuum-assisted breast biopsy. Eur Radiol 2014;24:1651-8. [Crossref] [PubMed]
- Chen SC, Yang H-R, Hwang T-L, et al. Intraoperative Ultrasonographically guided excisional biopsy or vacuum-assisted core needle biopsy for nonpalpable breast lesions. Ann Surg 2003;238:738-42. [Crossref] [PubMed]
- He XF, Ye F, Wen JH, et al. High residual tumor rate for early breast cancer patients receiving vacuum-assisted breast biopsy. J Cancer 2017;8:490-6. [Crossref] [PubMed]
- Chen P, Zhou D, Wang C, et al. Treatment and outcome of 341 papillary breast lesions. World J Surg. 2019;43:2477-82. [Crossref] [PubMed]
- Liao M, Huang J, Wu H, et al. Shall we take a second thought before applying radiofrequency ablation for resectable HCC ≤2 cm? Hepatobiliary Surg Nutr 2014;3:109-11. [PubMed]
- Ouyang Q, Li S, Tan C, et al. Benign phyllodes tumor of the breast diagnosed after ultrasound-guided vacuum-assisted biopsy: Surgical excision or wait-and-watch? Ann Surg Oncol 2016;23:1129-34. [Crossref] [PubMed]
- Sookoian S, Pirola CJ. Cell-free DNA methylation as liquid biopsy for the assessment of fibrosis in patients with nonalcoholic steatohepatitis: a gap between innovation and implementation. Hepatobiliary Surg Nutr 2017;6:117-21. [Crossref] [PubMed]
- Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci 2019;20. [PubMed]
- Pistolese CA, Ciarrapico AM, Della Gatta F, et al. Cost-effectiveness analysis of two vacuum-assisted breast biopsy systems: Mammotome and Vacora. Radiol Med 2009;114:743-56. [Crossref] [PubMed]
- Pistolese C, Castrignanò A, Ricci F, et al. Ultrasound-guided vacuum-assisted biopsy in small breast: A cost saving solution. Clin Breast Cancer 2019;19:e352-7. [Crossref] [PubMed]