
Lung cancer associated with cystic airspaces: CT and pathological features
Introduction
Lung cancer is one of the most common malignant tumors and remains the leading cause of cancer death worldwide. The awareness and early diagnosis of lung cancer have greatly improved in recent years thanks to the advances in computed tomography (CT) and video-assisted thoracoscopic surgery (VATS). However, missed and delayed diagnoses are still common due to the polymorphisms in lung cancer and the limited awareness and knowledge of this disease. A special type of lung cancer, lung cancer associated with cystic airspaces (LC-CAS), has a prevalence rate of about 3.6% (1), which is often misdiagnosed due to its similar imaging appearances to pulmonary bulla or pulmonary cyst.
According to the classification of Mascalchi, LC-CAS can be divided into four categories: type I, the nodule located outside the cystic cavity; type II, the nodule located inside the cystic cavity; type III, annular thickening of the cystic cavity wall; type IV, the multilocular cystic cavity mixed with solid or non-solid nodule. Because of the thin cystic cavity wall of LC-CAS, it is easy to cause pneumothorax or rupture of tumor body by CT-guiding puncture biopsy, so it is difficult to obtain ideal pathological tissue. What’s more, LC-CAS is mostly peripheral type, which leads to a low positive rate of sputum exfoliated cells and bronchoscopy. Therefore, it is of clinically significance to distinguish LC-CAS from benign tumors based on imaging technology, which will favor to improve the understanding, diagnosis and treatment of LC-CAS
Even though a recent study (2) has reported CT and pathological features of LC-CAS, it focused on the imaging features correlated with pathological invasiveness. In the current study, we analyzed the CT signs and clinicopathological features of LC-CAS patients in our hospital, and found that the most common pathological type of LC-CAS is adenocarcinoma, and the CT signs of LC-CAS were diverse.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1926).
Methods
A retrospective analysis was performed on 35 LC-CAS patients treated in our center from January, 2017, to January, 2020. There were 23 men and 12 women aged 61.4±9.2 years. CT showed that the cystic cavities were 1.6±0.7cm and ranged between 0.5 and 3.0cm in diameter. The CT signs of these lesions included lobulation sign (n=25), spicule sign (n=21), pleural indentation sign (n=22), vessel convergence sign (n=17), nodules on cystic wall (n=16), bronchial cut-off sign (n=15), uneven thickening of the cystic wall (n=10), and honeycombing (n=7). The lesions were central in 2 cases and peripheral in 33 cases, and were located in the left upper lung in 7 cases, the left lower lung in 9 cases, the right upper lung in 8 cases, the right middle lobe in 2 cases, and the right lower lobe in 9 cases. The morphological (3) types included type I (nodules located outside the cystic airspaces) in 11 cases (31.4%), type II (nodules located inside the cystic airspaces) in 9 cases (25.7%), type III (cyst wall thickening) in 8 cases (22.8%), and type III (multilocular cysts and the co-existence of solid and non-solid nodules) in 7 cases (20.0%).
All procedures performed in this study were in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of Zhejiang University Zhoushan Hospital. Because of the retrospective nature of the research, the requirement for informed consent was waived.
Results
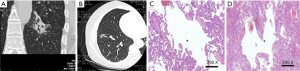
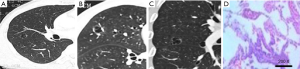
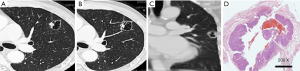
The lesions were adenocarcinomas in 30 patients, among which 22 were invasive adenocarcinoma (IACs); CT revealed uneven thickness or different density shadows on the inner wall of the cystic airspaces, and the proportion of ground-glass opacity nodules (GGNs) with solid component was higher than 30% (Figure 1). Minimally invasive adenocarcinoma (MIA) was detected in 8 cases, in whom CT revealed well-defined vacuolization-like translucent shadows; locally increased density of soft tissues was visible at the border, whereas the proportion of its solid component was below 30% (Figure 2). In addition, there were 4 cases of squamous cell carcinoma and 1 case of adenosquamous carcinoma; the lesions were manifested as single cystic airspace shadow in most cases, with a thick cystic wall; the nodules grew locally, and they typically protruded into the cystic airspace; notably, there were no surrounding GGNs (Figure 3).
Discussion
Although the description of LC-CAS can be traced back to 1941, there is still no widely recognized definition and nomenclature of this disease. Fintelmann et al. (4) believe that the cystic airspace refers to the pathological enlargement of the original cavities in lungs, and the solitary thin-walled air-containing cystic airspace is often located in lung parenchyma, with a clearly defined boundary. In most cases the cystic walls measured >8 mm. The Fleischner Society has a broader definition of LC-CAS (5); for example, lung cancer can arise from cystic cavities including emphysematous bullae and cysts. Several explanations have been proposed for the pathogenesis of LC-CAS (6-9): (I) tumor cells grow along the alveolar wall, and the damaged alveolar wall fuses to form cystic airspaces; (II) after liquefaction and necrosis, the space-occupying lesions are discharged to form cystic airspaces; (III) the elastic retraction of the tissues around the lung causes the cavity to be pulled, causing the cystic wall to become thin.
A fourth explanation involves the cystic cavity arising from the “valve effect”, in which the tumor cells originate from the bronchiolar epithelium, the tumor grows, the bronchioles are completely blocked, and the dilation and rupture of the distal alveoli subsequently occur. As a result, the inner wall of the cavity and the nodules in the outer wall form, and the annular wall of cyst thickens. The valve effect has been widely recognized, and we also hold that the valve effect is one of the main pathogenic mechanisms for LC-CAS. The effect is perhaps even more obvious when the fragility of the alveolar space increases as the tumor grows, and when the stretching of the tissues outside the bronchi and the changes in the structure of the bronchial wall also cause irregular bronchiectasis. The tumor tissue has its internal traction. The partially blocked bronchioles contribute to the formation of valves and thus cystic airspaces. A better understanding of the origin of LC-CAS can also explain why LC-CASs are predominantly peripheral.
Many studies have described the pathological types of LC-CAS. Liu et al. (10) reported that all 26 of their patients had peripheral LC-CAS; in addition, most of the lesions were IACs. Kelley et al. (11) reported that squamous cell carcinoma accounted for about 47.8% of LC-CAS cases. Other literature focuses (12-16) on different pathological types including squamous cell carcinoma, large cell carcinoma, and small cell carcinoma in LC-CAS patients; nevertheless, the vast majority of LC-CAS shave been reported to be adenosquamous carcinoma, with adenocarcinoma being the most common type. Among the 35 cases in our current series, the pathological types included adenocarcinoma (n=30, accounting for 85.7%), squamous cell carcinoma (n=4), and adenosquamous carcinoma (n=1), which were similar to the results reported in the literature. We also found 22 cases of IAC and 8 cases of MIA. Although the proportion of IAC was high, we speculate that MIA may be more common than IAC when considering the pathogenic mechanism of LC-CAS, but this needs to be further investigated in larger patient cohorts.
It is difficult to obtain ideal pathological tissue due to the cystic changes of LC-CAS, but CT three-dimensional reconstruction can clearly display the microstructures inside and outside the wall of cystic airspaces. As a non-invasive examination, it is cheaper than positron emission tomography-CT (PET-CT) and is more feasible for follow-up and observations. The CT signs of the lesions in our series included lobulation sign (n=25, 71.4%), spicule sign (n=21, 62.8%), and pleural indentation sign (n=22, 57.1%). According to the method of Mascalchi et al., the proportions of LC-CAS types in our current series included type I (31.4%), type II (25.7%), type III (22.8%), and type IV (20.0%). Thus, LC-CAS has diverse imaging features, which include both the common CT signs of lung cancer and other more characteristic findings such as the rough and honeycombed inner wall of the cystic spaces, nodules on the wall, and vascular bundles. Among them, CT of IAC showed cystic translucent shadows: the thickness of the inner wall of the cystic airspaces was uneven, the airspaces were surrounded by GGNs or solid components, and the proportion of solid components accounted was greater than 30%. The proportion of the shadow of cystic airspaces was lower in IAC than in MIA, which might be explained by the fact that the cystic airspaces were replaced by solid components. In eight patients, MIA showed coelentereous growth, along with mucous components. CT revealed well-defined vacuolization-like translucent shadows: locally increased density of soft tissues was visible at the border, whereas the proportions of its solid component and GGNs were below 30%. Unlike the traditional belief that squamous cell carcinoma is prone to form cavities, these lesions are known to us as cystic squamous cell carcinoma. Typically, the cystic wall of cystic squamous cell carcinoma is thicker than that of adenocarcinoma; nodules on the cystic wall are caused by tumor growth rather than by residues after tumor necrosis. Necrosis in the cystic wall is not obvious. Typically there is only 1 cyst. Difference in growth makes the cystic wall irregularly thickened locally. The lesions were manifested as single cystic airspace shadow in most cases, and there was a thick cystic wall. The nodules grew locally, and they typically protruded into the cystic airspace, with, notably, no surrounding GGNs. It is therefore speculated that the proportions of pericystic GGNs, mixed GGNs, and solid components somehow reflect the pathological characteristics of a tumor, and the chaotic internal structures of cystic airspaces may suggest its malignant property. The solid nodules typically have rough borders in a peripheral lung cancer, along with the presence of lobulation sign, spicule sign, and pleural indentation sign. In contrast, benign cystic airspace-like lesions (e.g., pulmonary bullae) are mostly located in the air-containing cavities at the apex of the lung; these solitary or multiple lesions have smooth borders, and their internal structures have even density and show no abnormal change in density. Most patients with tuberculous cavities have the toxic symptoms of tuberculous infection or have a medical history of tuberculosis. The cavities often occur in the apical and posterior segments of the upper lobe and the dorsal segment of the lower lobe. The morphologies of these cavities are diverse, and satellite lesions can be seen around the cavities.
There are still some limitations in the current study. Firstly, the case enrolled in this study is small. Secondly, it is a single-center, retrospective study. A multiple-center, perspective study with more cases enrolled are required to further investigate the clinical manifestations of LC-CAS, so as to improve the accuracy of early diagnosis and reduce missed and delayed diagnoses.
In summary, LC-CAS is a special type of lung cancer. It has a low prevalence rate, with adenocarcinoma being its main pathological type. The CT signs of LC-CAS are diverse, and therefore CT-based follow-up and increased awareness and knowledge of this disease among clinicians are particularly important.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1926
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1926
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1926). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Zhejiang University Zhoushan Hospital. Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vlahos I, Stefanidis K, Sheard S, et al. Lung cancer screening: nodule identification and characterization. Transl Lung Cancer Res 2018;7:288-303. [Crossref] [PubMed]
- Snoeckx A, Reyntiens P, Carp L, et al. Diagnostic and clinical features of lung cancer associated with cystic airspaces. J Thorac Dis 2019;11:987-1004. [Crossref] [PubMed]
- Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr 2015;39:102-8. [Crossref] [PubMed]
- Fintelmann FJ, Brinkmann JK, Jeck WR, et al. Lung Cancers Associated With Cystic Airspaces: Natural History, Pathologic Correlation, and Mutational Analysis. J Thorac Imaging 2017;32:176-88. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Singh N, Bal A. Lung cyst caused by centrally located bronchogenic carcinoma. Arch Bronconeumol 2012;48:99-101. [PubMed]
- Lan CC, Wu HC, Lee CH, et al. Lung cancer with unusual presentation as a thin-walled cyst in a young nonsmoker. J Thorac Oncol 2010;5:1481-2. [Crossref] [PubMed]
- Iwata T, Nishiyama N, Nagano K, et al. Squamous cell carcinoma presenting as a solitary growing cyst in lung: a diagnostic pitfall in daily clinical practice. Ann Thorac Cardiovasc Surg 2009;15:174-7. [PubMed]
- He TP, Yang CB. Spiral CT diagnosis of lung cancer associated with cystic airspaces. Modern Medical Imagelogy 2012;21:303-7.
- Liu Z, Zhang ZR, Sun HL, et al. CT and clinicopathological features of thin-walled cystic lung cancer with the largest diameter less than or equal to 3 cm. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2019;26:
- Kelley KD, Benninghoff DL, Stein JS, et al. Medically inoperable peripheral lung cancer treated with stereotactic body radiation therapy. Radiat Oncol 2015;10:120. [Crossref] [PubMed]
- Usui S, Minami Y, Shiozawa T, et al. Differences in the prognostic implications of vascular invasion between lung adenocarcinoma and squamous cell carcinoma. Lung Cancer 2013;82:407-12. [Crossref] [PubMed]
- Lei Y, Wu Y. The prognostic value of micrometastasis in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2013;16:492-8. [PubMed]
- Igai H, Tarumi S, Chang SS, et al. Predictor of intrathoracic lymph node metastasis in peripheral non-small cell lung cancers 20 mm or less in greatest dimension. Kyobu Geka 2012;65:175-83. [PubMed]
- Watanabe Y, Kusumoto M, Yoshida A, et al. Cavity Wall Thickness in Solitary Cavitary Lung Adenocarcinomas Is a Prognostic Indicator. Ann Thorac Surg 2016;102:1863-71. [Crossref] [PubMed]
- Wang Y, Fan L, Liu SY. Advances in imaging and pathological studies on cystic airspaces-containing peripheral lung cancer. Journal of Clinical Radiology 2016;35:486-9.


