
NOVA1 expression is associated with clinicopathological characteristics and prognosis in patients with small cell lung cancer
Introduction
Lung cancer ranks the most common cancer and the leading cause of cancer-related death in the world (1). Small cell lung cancer (SCLC) is a deadly malignancy accounting for 13–15% of all lung cancer cases (2). As one kind of aggressive high-grade neuroendocrine tumors, SCLC is distinguished by rapid tumor proliferation, extensive metastatic dissemination and inevitably relapse (3), which leads to extremely poor clinical prognosis (lower than 7% five-year survival) (4). To date, plenty of studies have focused on the molecular pathogenesis and therapeutic options for patients with advanced non-small cell lung cancer (NSCLC) especially lung adenocarcinoma, such as customized chemotherapy and targeted specific treatments including bevacizumab, gefitinib, etc. (5,6). However, there are few novel therapeutic options for SCLC. Nowadays, platinum-based chemotherapy remains the first-line approach for SCLC whereas most patients have not achieved substantial clinical benefits (7). Therefore, detection and investigation of associated biomarkers of SCLC appear promising, which may potentially benefit therapy for patients with such disease.
Recently, RNA-binding proteins (RBPs) have been increasingly recognized as important post-transcriptional regulators which promote the development of diverse disorders including cancers (8,9). Neuro-oncological ventral antigen-1 (NOVA1), a neuron-specific RBP, can manipulate ligand-binding, electrophysiological and signal transducing properties (10). Growing data have demonstrated that the dysregulation of NOVA1 also plays a crucial role in the progression of various tumors such as hepatocellular carcinoma, melanoma and osteosarcoma (11-13). Nonetheless, it is still uncertain whether the expression of NOVA1 is relevant to clinicopathological features and prognosis in SCLC.
In the current study, we analyzed NOVA1 expression in SCLC tissues by immunochemistry, investigated the association of NOVA1 expression with clinicopathological markers and survival time of SCLC patients. We intended to elucidate the clinical role of NOVA1 in SCLC. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2806).
Methods
Patients and tissue specimens
The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai East Hospital, Tongji University School of Medicine (No. YS2020047). Written consents were obtained from all patients in this research. A total of 84 patients (47 males and 37 females; the average age was 68.13 years old) pathologically diagnosed with new primary SCLC from August 2012 to February 2016 at Shanghai East Hospital were randomly enrolled in the study. Clinicopathological characteristics of all the patients were detailed in Table 1. The histopathologic diagnosis was based on the samples obtained by surgical resection, bronchoscopy, thoracoscopy, or percutaneous lung biopsy. The tumor staging criteria is accordance with the Veterans Administration Lung Cancer Study Group (VALSG) staging system (14). Presence or absence of brain metastases was assessed through PET/CT scanning or magnetic resonance examination. Clinical manifestations of paraneoplastic neurological syndrome (PNS) were judged by the physical examination, laboratory test, electromyography, electroencephalogram, etc. according to recommended diagnostic criteria (15). All patients were followed up every 3–4 months afterward. During follow-up evaluations, all cases were prospectively observed by serum NSE, chest computed tomography (CT) and/or brain magnetic resonance imaging (MRI) scanning. Overall survival time was defined from the diagnosis time to the death time or the end of follow-up.
Table 1
| Variables | NOVA1 expression | χ2 | P value | |
|---|---|---|---|---|
| Low | High | |||
| Age | 0.065 | 0.487 | ||
| <60 y | 16 | 20 | ||
| ≥60 y | 20 | 28 | ||
| Gender | 0.258 | 0.387 | ||
| Male | 19 | 28 | ||
| Female | 17 | 20 | ||
| Smoking status | 0.219 | 0.409 | ||
| Present | 25 | 31 | ||
| Absent | 11 | 17 | ||
| Tumor size | 0.875 | 0.483 | ||
| <3 cm | 10 | 18 | ||
| ≥3 cm | 26 | 30 | ||
| Tumor staging | 15.833 | <0.001 | ||
| Limited stage | 20 | 7 | ||
| Extensive stage | 16 | 41 | ||
| Lymph node metastasis | 16.852 | <0.001 | ||
| Present | 10 | 35 | ||
| Absent | 26 | 13 | ||
| Brain metastasis | 9.624 | 0.002 | ||
| Present | 31 | 26 | ||
| Absent | 5 | 22 | ||
| PNS | 5.004 | 0.024 | ||
| Present | 1 | 4 | ||
| Absent | 35 | 44 | ||
SCLC, small cell lung cancer; PNS, paraneoplastic neurological syndromes; NOVA1, neuro-oncological ventral antigen-1.
Immunohistochemistry
All tissue specimens were immediately formalin-fixed for 24–48 h and then embedded in paraffin. Four µm sections were collected on coated slides and processed for immunohistochemistry. Firstly, Sections were dropped with 3% hydrogen peroxide solution and incubated at room temperature for 10 min. After microwave antigen retrieval for 20 min, the sections were dropped with normal goat serum and incubated at room temperature for 20 min. Then they were dropped with anti-NOVA1 antibody (1:500 concentration; EPR13848; Abcam) and incubated at 4 °C overnight. On the next day, they were dropped with biotin-labeled secondary antibody and incubated at 37 °C for 15 min. Last, the sections were developed using freshly prepared DAB for 3–5 min, and observed under a microscope, with positive cells displaying brown staining or positive cells displaying brown granules. The sections were routinely dehydrated, cleared and finally sealed.
Assessment of NOVA1 staining and scoring
Under one optical microscope (Olympus BX43, Japan), tissue expression of NOVA1 was analyzed by two pathologists using Friedrichs scoring system. The staining intensity was scored as four grades, “0” for no staining, “1” for weakly stained, “2” for moderately stained, “3” for deeply stained. The percentage of NOVA 1 staining positive cells were ranked into score “0” for less than 10%, “1” for 11–50%, “2” for 51–75% and “3” for more than 75%. The total immunoreactivity scores were recorded by multiplying the quantity scores of the staining intensity and the percentage of NOVA 1 staining positive cells. Expression level of NOVA1 is divided into two grades: low expression (total score <4) and high expression (total score ≥4), respectively.
Statistical analysis
Statistical analysis was performed using the SPSS17.0 software. The immunoreactive scores of NOVA1 was expressed as mean ± standard deviation (SD). Student’s t-test and analysis of variance (ANOVA) were used for comparisons in different groups. The correlations between NOVA1 expression level and clinicopathological features were calculated by the Pearson chi-square χ2 test. The Cox proportional hazard regression model was applied to search for prognostic factors contributing to survival. Survival curves were established using the Kaplan-Meier method, the differences between which were calculated with the log-rank test. P value <0.05 was considered statistically significant and all the confidence intervals (CIs) were at the 95% level.
Results
The expression of NOVA1 in cancerous tissues of SCLC patients and normal lung tissues
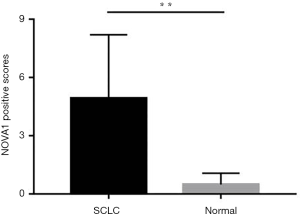
The expressions of NOVA1 in cancerous tissues in 84 cases of SCLC and adjacent normal lung tissues were first detected. Histopathological findings revealed that tumor cell nuclei showed widespread positivity for NOVA1 in the 84 cases of cancerous tissues while almost none cells exhibited positive immunoreactivity in adjacent normal tissues (Figure 1). The scores of NOVA1+ expressed cells (judging by percentage of NOVA1 positive cells as well as staining intensity) per field in tumorous tissues were distinctively higher than those in adjacent normal tissues. The difference between the two groups is statistically of significance (scores of NOVA1 expression: 6.94±2.27 vs. 0.42±0.59, P<0.01) (Figure 2).
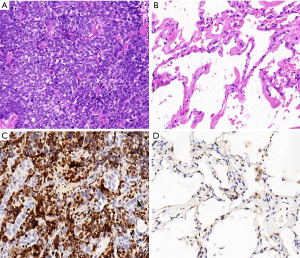
Relationship between tissue NOVA1 expression and clinicopathological characteristics
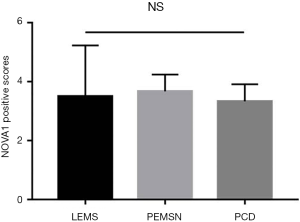
According to the grading of immunoreactivity scores of NOVA1 expression, there were 48 cases categorized as high expression of NOVA1 and 36 cases as low expression of NOVA1, respectively (Figure 3). The clinicopathological characteristics of 84 SCLC patients were also summarized in Table 1. Through Pearson chi-square test method, the results demonstrated that tissue expression of NOVA1 was significantly correlated with tumor staging (χ2=15.833, P<0.001), lymph node metastasis (χ2=16.852, P<0.001), brain metastasis (χ2=9.624, P=0.002) and PNS (χ2=5.001, P=0.024). Of the patients studied, PNS were recorded in 10 cases (11.9%) (Table 2). Furthermore, we analyzed the characteristics of these patients presented with PNS, finding that there were 4 cases showing Lambert-Eaton myasthenic syndrome (LEMS), 3 cases showing paraneoplastic encephalomyelitis sensory neuronopathy (PEMSN), and 3 case showing paraneoplastic cerebellar degeneration (PCD) (Table 3). Of note, although NOVA1 expression was correlated with PNS, immunoreactivity scores of NOVA1 expression had yet no significant difference among the three types of paraneoplastic neurological disorders (shown in Figure 4).
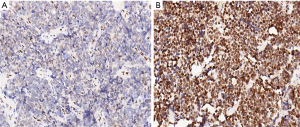
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥60 vs. <60 y) | 1.164 (0.661–2.051) | 0.599 | |||
| Gender (male vs. female) | 1.032 (0.589–1.809) | 0.911 | |||
| Smoking status (present vs. absent) | 0.807 (0.452–1.441) | 0.468 | |||
| Tumor size (≥3 vs. <3 cm) | 0.667 (0.369–1.205) | 0.190 | |||
| Tumor staging (extensive vs. limited) | 7.871 (3.726–16.630) | <0.001 | 0.272 (0.102–0.727) | 0.009 | |
| Lymph node metastasis (present vs. absent) | 8.590 (3.889–18.974) | <0.001 | 0.317 (0.129–0.777) | 0.012 | |
| Brain metastasis (present vs. absent) | 5.675 (2.847–11.313) | <0.001 | 0.428 (0.198–0.925) | 0.031 | |
| PNS (present vs. absent) | 2.315 (1.028–5.214) | 0.043 | 0.495 (0.197–1.243) | 0.134 | |
| NOVA1 expression (low vs. high) | 0.193 (0.100–0.372) | <0.001 | 0.445 (0.213–0.934) | 0.032 | |
SCLC, small cell lung cancer; PNS, paraneoplastic neurological syndromes; NOVA1, neuro-oncological ventral antigen-1; CI, confidence interval.
Table 3
| No. | Age | Gender | PNS | NOVA1 immunoreactivity scores | Tumor staging |
|---|---|---|---|---|---|
| 1 | 63 | Male | LEMS | 2 | Limited |
| 2 | 68 | Female | LEMS | 3 | Extensive |
| 3 | 66 | Male | PEMSN | 4 | Extensive |
| 4 | 54 | Male | LEMS | 6 | Extensive |
| 5 | 72 | Male | PCD | 3 | Extensive |
| 6 | 63 | Female | LEMS | 3 | Extensive |
| 7 | 70 | Female | PEMSN | 4 | Extensive |
| 8 | 62 | Male | PEMSN | 3 | Limited |
| 9 | 58 | Male | PCD | 3 | Extensive |
| 10 | 69 | Male | PCD | 4 | Extensive |
SCLC, small cell lung cancer; PNS, paraneoplastic neurological syndromes; LEMS, Lambert-Eaton myasthenic syndrome; PEMSN, paraneoplastic encephalomyelitis sensory neuronopathy; PCD, paraneoplastic cerebellar degeneration.
Survival analysis
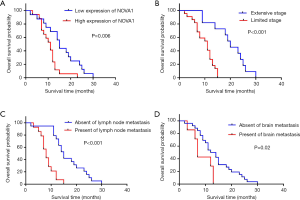
The median follow-up time was 25.1 months (ranging from 2.0 to 63.9 months). By February 2019, the end of the follow-up, only 4.7% patients were alive. With the COX proportional hazard regression model, advanced tumor stage (HR =0.272; 95% CI: 0.102–0.727; P=0.009), high expression of NOVA1 (HR =0.445; 95% CI: 0.213–0.934; P=0.032) as well as lymph node (HR =0.317; 95% CI: 0.129–0.777; P=0.012) and brain metastasis (HR =0.428; 95% CI: 0.198–0.925; P=0.031) were independent factors for shorter survival time(shown in Table 2). Kaplan-Meier method was further applied to analyze overall time according to independent prognostic factors. The mean and median overall survival time of patients with low NOVA1 expression were longer than those with high NOVA1 expression according to log-rank test (shown in Table 4 and Figure 5).
Table 4
| Variable | Mean (SE) (95% CI) | Median (SE) (95% CI) | P value |
|---|---|---|---|
| NOVA1 | 0.006 | ||
| High | 12.16 (1.58) (10.02–13.31) | 12.00 (1.12) (9.81–14.19) | |
| Low | 20.74 (1.89) (17.02–24.46) | 16.00 (0.84) (14.35–17.65) |
SCLC, small cell lung cancer; NOVA1, neuro-oncological ventral antigen-1; CI, confidence interval.
Discussion
SCLC is a lethally malignant lung cancer subtype with a short doubling time and early progress of extensive metastases, which poses great challenges to effective therapeutic procedures (7,16). In the past decades, the anti-tumor strategies for SCLC have not been changed due to aggressive propensity of this disease and the lack of predictive biomarkers of clinical efficacy.
NOVA1 has been suggested as a novel RBP that participates in the tumorigenesis of multiple cancers (17). Based on these observations, we retrospectively analyzed the association between NOVA1 expression and clinicopathological features in patients with SCLC on a relative large sample scale in the present study. Our findings exhibited that NOVA1 was remarkably expressed in SCLC tumor tissues compared with adjacent normal lung tissues, indicating that NOVA1 may specifically exert an influence on the progression of SCLC. Besides, in previous work, our team also found the mRNA level of NOVA1 expression was higher in the SCLC H446 cells than that in normal BEAS-2B bronchial epithelial cells and lung adenocarcinoma A549 or H226 SCC cells. We thought NOVA1 was specifically expressed in SCLC cell lines as well (18). In accordance with previous data (5), our study revealed that the average 5-year survival rate of the 84 patients with SCLC was terribly low (just 4.7%). We also found that over-expression of NOVA1 was closely related to poor survival, suggesting that NOVA1 is a potential biomarker in predicting cancer-specific prognosis in patients with SCLC. Although emerging researches have confirmed that NOVA1 acts as an oncogene due to its contribution to the development of various tumors, this is the first study focusing on the association of NOVA1 with clinicopathological characteristics in patients with SCLC. Interestingly, our data showed that not tumor size but tumor staging and lymph node metastasis were greatly correlated with tissue expression level of NOVA1. We hypothesized that NOVA1 overexpression may play a preferable role in tumor metastasis and invasion rather than proliferation in SCLC. Further research will be required to verify this view. Because of highly malignant potency of SCLC and rare well-differentiated tumor cells discovered in the measured tissue samples, we herein did not take pathological grade into consideration in this study.
As an aggressive neuroendocrine carcinoma, SCLC is specially characterized by the profiles of PNS. PNS is described as tumor-related neurological disorders that have remote damage effects on neuronal tissues (19). Moreover, SCLC-related PNS is essentially a unique antoimmune response in which several highly specific onconeural autoantibodies and autoantigens involved (20). The role of NOVA1 as a SCLC-associated antoantigen has been reported in some researches (21,22), whereas the relationship of NOVA1 expression and PNS in SCLC remains limited. Similar to previous ligeratures (23), our study showed totally 10 patients (11.9%) presented clinical manifestations of PNS in which LEMS was the most common form. Besides, we observed the prevalence of PNS were 18.7% (9/48) in cases with high NOVA1 expression while 2.8% (1/36) in those with low NOVA1 expression, with a statistic difference between them. Hence, we assumed that NOVA1, the SCLC-associated autoantibody, may critically participate in the pathogenesis of PNS in SCLC. More importantly, we have analyzed the clinicopathological characteristics of those patients with PNS and evaluated the correlations of NOVA1 with diverse PNS manifestations. Of note, the expression of NOVA1 in tumorous tissues had no significant difference among the three groups with different types of SCLC-associated PNS (LEMS, PEMSN, PCD, respectively) in our cohort. There are two points that may account for such finding. Firstly, the number of patients suffering PNS was really small, probably limiting the assessment on the utility of NOVA1 amongst different types of PNS. Secondly, previous work has investigated that NOVA1 is an important autoimmune antigen in paraneoplastic opsoclonus-myoclonus ataxia (POMA) (24). In the present study, however, no patient demonstrated the clinical manifestation of POMA. Thus, it is likely that NOVA1 is not the specific PNS-associated antigen in the process of LEMS, PEMSN as well as PCD. Currently, at least 20 antigens have been known in SCLC-associated PNS such as HuD, VGCC, SOX etc. (25). For instance, all SCLC tumors express HuD protein. Furthermore, the HuD-specific cytotoxic T cell plays a crucial role in the pathogenesis of SN by initiating immune response (26). Voltage-gated calcium channels (VGCCs), a transmembrane protein, is another common antigen that highly associates with the development of LEMS (27). Whether NOVA1 can be served as a useful antigen for predicting some type of PNS should be further explored.
We also evaluated the contribution of clinicopathological parameters to survival prognosis of patients with SCLC using a Cox proportional hazard model. The result demonstrated that SCLC patients with high NOVA1 expression intended to have shorter survival, confirming the role of NOVA1 as an independent prognostic factor in SCLC overall survival. In addition, tumor staging, lymph node and brain metastasis were also considered to be significant indicators for poor prognosis. Currently, some reports have suggested that SCLC patients with PNS have longer survival times because of the efficient antitumor immune response (28,29). Nevertheless, other reports haven’t found any evidence on the relationship between PNS and prognosis benefits in SCLC (30). In this study, we found that PNS did not appear to be correlated with survival, indicating that the predictive value of PNS for prognosis in SCLC is still equivocal. The role of PNS in assessment of prognosis of SCLC needs to be scrutinized on a larger sample size of multicenter studies.
Last but not the least, certain limitations of our research should be noted. In the present study, notwithstanding most tissue specimens were obtained in small biopsies, there were a few surgical excision tissues from some limited-stage SCLC patients. This discrepancy might affect the evaluation of tissue NOVA1 expression, which possibly renders the results biased. The detailed function of NOVA1 in SCLC may be further elucidated according to standardized tissue materials.
Conclusions
To summarize, our results shed light on the relationship between NOVA1 tissue expression and clinicopathological characteristics of patients with SCLC. NOVA1 is proved to be a promising biomarker for indicating survival prognosis and probably a novel therapeutic target. Our study may provide some theoretical basis for molecular pathogenesis and clinical treatment of SCLC.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2806
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2806
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-19-2806
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2806). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai East Hospital, Tongji University School of Medicine (No. YS2020047). Written consents were obtained from all patients in this research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-236. [Crossref] [PubMed]
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628-38. [PubMed]
- Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol 2001;28:3-13. [Crossref] [PubMed]
- Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:300-11.
- Hong J, Kyung SY, Lee SP, et al. Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small cell lung cancer. Korean J Intern Med 2010;25:294-300. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Jett JR, Schild SE, Kesler KA, et al. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e400S-e419S.
- Neelamraju Y, Gonzalez-Perez A, Bhat-Nakshatri P, et al. Mutational landscape of RNA-binding proteins in human cancers. RNA Biol 2018;15:115-29. [Crossref] [PubMed]
- Dassi E. Handshakes and Fights: The Regulatory Interplay of RNA-Binding Proteins. Front Mol Biosci 2017;4:67. [Crossref] [PubMed]
- Buckanovich RJ, Posner JB, Darnell RB. Nova, the paraneoplastic Ri antigen, is homologous to an RNA-binding protein and is specifically expressed in the developing motor system. Neuron 1993;11:657-72. [Crossref] [PubMed]
- Zhang YA, Zhu JM, Yin J, et al. High Expression of Neuro-Oncological Ventral Antigen 1 Correlates with Poor Prognosis in Hepatocellular Carcinoma. PLoS One 2014;9,3:e90955.
- Yu X, Zheng H, Chan MTV, et al. NOVA1 acts as an oncogene in melanoma via regulating FOXO3a expression. J Cell Mol Med 2018;22:2622-30. [Crossref] [PubMed]
- Li C, He Y, Ma H, et al. NOVA1 acts as an oncogene in osteosarcoma. Am J Transl Res 2017;9:4450-7. [PubMed]
- Carter BW, Glisson BS, Truong MT, et al. Small Cell Lung Carcinoma: Staging, Imaging, and Treatment Considerations. Radiographics 2014;34:1707-21. [Crossref] [PubMed]
- Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135-40. [Crossref] [PubMed]
- Sculier JP, Berghmans T, Castaigne C, et al. Maintenance chemotherapy for small cell lung cancer: a critical review of the literature. Lung Cancer 1998;19:141-51. [Crossref] [PubMed]
- Xin Y, Li Z, Zheng H, et al. Neuro-oncological ventral antigen 1 (NOVA1): Implications in neurological diseases and cancers. Cell Prolif 2017;50. [PubMed]
- Deng S, Liu M, Xiao T, et al. Expression of neuro-oncological ventral antigen-1 in small-cell lung cancer and its value in pathological diagnosis. Transl Cancer Res 2020;9:1144-50. [Crossref]
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003;349:1543-54. [Crossref] [PubMed]
- Toothaker TB, Rubin M. Paraneoplastie neurological syndromes: a review. Neurologist 2009;15:21-33. [Crossref] [PubMed]
- Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol 2004;56:715-9. [Crossref] [PubMed]
- Buckanovich RJ, Yang YY, Darnell RB. The onconeural antigen Nova-1 is a neuron-specific RNA-binding protein, the activity of which is inhibited by paraneoplastic antibodies. J Neurosci 1996;16:1114-22. [Crossref] [PubMed]
- Titulaer MJ, Wirtz PW, Willems LN, et al. Screening for small-cell lung cancer: a follow-up study of patients with Lambert-Eaton myasthenic syndrome. J Clin Oncol 2008’26:4276-81.
- Yang YY, Yin GL, Darnell RB. The neuronal RNA-binding protein Nova-2 is implicated as the autoantigen targeted in POMA patients with dementia. Proc Natl Acad Sci U S A 1998;95:13254-59. [Crossref] [PubMed]
- Kazarian M, Laird-Offringa IA. Small-cell lung cancer-associated autoantibodies: potential applications to cancer diagnosis, early detection, and therapy. Mol Cancer 2011;10:33. [Crossref] [PubMed]
- Voltz R, Dalmau J, Posner JB, et al. T-cell receptor analysis in anti-Hu associated paraneoplastic encephalomyelitis. Neurology 1998;51:1146-50. [Crossref] [PubMed]
- Wirtz PW, Lang B, Graus F, et al. P/Q-type calcium channel antibodies, Lambert-Eaton myasthenic syndrome and survival in small cell lung cancer. J Neuroimmunol 2005;164:161-5. [Crossref] [PubMed]
- Maddison P, Gozzard P, Grainge MJ, et al. Long-term survival in paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 2017;88:1334-9. [Crossref] [PubMed]
- Gozzard P, Chapman C, Vincent A, et al. Novel humoral prognostic markers in small-cell lung carcinoma: A prospective study. PLoS One 2015;10:e0143558. [Crossref] [PubMed]
- Payne M, Bradbury P, Lang B, et al. Prospective study into the incidence of Lambert Eaton myasthenic syndrome in small cell lung cancer. J Thorac Oncol 2010;5:34-8. [Crossref] [PubMed]


