
Comparison of the proliferative and clonogenic growth capacity of wound fluid from breast cancer patients treated with and without intraoperative radiotherapy
Introduction
The ability of the cellular microenvironment to influence cell behavior has been known for quite some time. In malignancies, the microenvironment was shown to regulate tumor cell fate even suggesting that disruption of its homeostasis may drive tumor progression (1). Induction of the wound healing response after surgery with the ensuing microenvironment reorganization and tissue reconstruction may, therefore, potentially influence recurrence (2). After damage, wound healing, including inflammation, tissue repair, and remodeling, is essential to ensure host integrity in multicellular eukaryotic organisms (3). As previously shown in experimental systems (4,5), it is to be expected that growth factors secreted during wound healing can also affect growth of malignant and non-malignant cells clinically. It was previously observed that wound fluid (drained after surgery; WF) collected from breast cancer patients can indeed stimulate proliferation of breast cancer cells (6).
In intraoperative radiotherapy (IORT), a high single dose of radiation is applied to the tumor bed directly after surgical removal of the tumor, in contrast to conventional external-beam radiotherapy (EBRT) which is applied after wound healing is completed. In a previous study, WF obtained from patients treated with IORT within the TARGIT-A trial (7) was reported to produce a reduction in WF-stimulated proliferation and invasion of breast cancer cell lines in vitro compared to WF from non-IORT patients (8,9). However, no significant effect of IORT on the proliferative capacity of WF was observed in a short-term proliferation assay (2-D) using ER/PgR−-Her2/neu− and ER/PgR−-Her2/neu+ breast cancer cell lines, Furthermore, although significant effects of IORT were found in invasion (3-D Matrigel) and migration assays, clonogenic proliferation was not tested.
Therefore, the purpose of the present study was to validate the effect of IORT on WF-stimulated short-term proliferation in an ER/PgR+-Her2/neu- human breast cancer cell line (MCF7), and for the first time test the effect on clonogenic, long-term proliferation.
Methods
Cell culture
The human breast carcinoma cell line MCF7 (ER/PgR+-Her2/neu−; American Type Culture Collection, LGC Standards GmbH, Wesel, Germany) was propagated in DMEM supplemented with 10% fetal bovine serum (FBS; all from Biochrom AG, Berlin, Germany). Three days before each experiment, cells were cultured in 3% FBS-containing medium. Cells were kept at 37 °C in a humidified incubator with 95% air/5% CO2.
Collection and preparation of WF
Thirty patients with low-risk breast cancer were treated with breast-conserving surgery, of which 12 received IORT with a single dose of 20 Gy prescribed to the applicator surface (10-12). After surgery, WF was drained from the wound for 24 h. Thereafter, WF samples were collected, centrifuged at 800 ×g for 5 min and the supernatant was filtered through 40 µm filters (BD Falcon, Heidelberg, Germany). After a second centrifugation step (3,500 ×g for 5 min), the supernatant was subsequently filtered through 5, 0.8 and 0.22 µm filters and aliquots stored at −80 °C. These steps ensured sufficient removal of cells and debris from the WF that would otherwise interfere with cell growth. The study was approved by the Medical Ethics Commission II of the Medical Faculty of Mannheim, Heidelberg University and was conducted according to Declaration of Helsinki principles. Of note, although all patients received a perioperative antibiotic treatment, this was prolonged (3 days) for patients receiving IORT vs. a single application for non-IORT-treated patients.
Proliferation assay
MCF7 cells were seeded in 96 well plates (8 wells per group, 5×103 cells per well in 50 µL serum-free DMEM medium). Samples were supplemented with 50 µL DMEM medium containing 3% FBS and 1% or 3% WF. After 48 h, 20 µL MTT [5 mg/mL, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to each well and incubated for 3 h at 37 °C. Viable cells reduce the yellow MTT to a non-hydrosoluble purple formazan. Thereafter, 100 µL of 10% SDS (Sodium dodecyl sulfate)/10 mM HCl in PBS was added to each well and plates incubated o/n at 37 °C to allow dissolving of the formazan. The next day, the absorbance at 590 nm (reference 690 nm) was quantified using a spectrophotometer (Tecan Infinite M200). Results are shown as percentage of controls.
Colony formation assay
MCF7 cells were seeded at 150 cells/T25 culture flask (triplicates) in 4 mL DMEM medium supplemented with 3% FBS and 3% WF and incubated for 2 weeks in a humidified incubator with 95% air/5% CO2 at 37 °C. Thereafter, cells were fixed with methanol/acetic acid and stained with crystal violet as described previously (13). Colonies (≥50 cells) were scored and the plating efficiency determined: plating efficiency = number of colonies obtained/number of cells seeded.
Statistics
Replicates were performed at least in triplicate and data are presented as mean ± standard error, unless otherwise noted. Wilcoxon/Kruskal-Wallis (non-parametric) tests and linear regression were performed with JMP11 statistical software (SAS Institute GmbH, Böblingen, Germany). Graphs were plotted using SigmaPlot 11.0 (Systat Software GmbH, Erkrath, Germany).
Results
Effects of IORT on the short-term proliferative capacity of WF
Pilot experiments showed that MCF7 cells did not proliferate when supplemented with pure WF and that 3% FBS was required to warrant proper proliferation (data not shown). To test potential concentration effects, experiments were performed with either 1% or 3% WF added to medium containing 3% FBS and normalized to the proliferation rates of cells receiving only 3% FBS. For 1% WF a trend for a modest inhibiting effect of IORT on the proliferative capacity of WF was observed (P=0.07; no IORT: 101.3%±4.0% vs. IORT: 91.0%±3.0%; Figure 1A) and the difference was also not significant using 3% WF (P=0.16; no IORT: 97.4%±2.8% vs. IORT: 92.0%±3.6%; Figure 1B). As large variations in the volumes of the WF occurred, a potential correlation between the volume and the effect on proliferation of MCF7 cells was tested. Here, no correlation could be detected, irrespective of using 1% (R2No IORT=0.138, R2IORT=0.119; Figure 1C) or 3% WF (R2No IORT=0.045, R2IORT=0.015; Figure 1D).
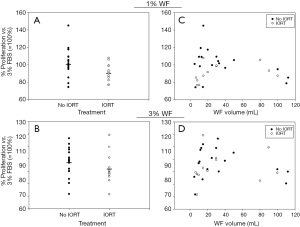
Effects of IORT on the clonogenic growth capacity of WF
As repopulation of residual tumor cells to form recurrences depends on the capacity of cells to reproduce themselves, the effect of IORT on WF-stimulated clonogenic growth was tested in the colony formation assay. 3% WF from patients receiving IORT had no significant effect on the plating efficiency after 14 days incubation compared to that of WF from patients not receiving IORT (P=0.79; no IORT: 29.0%±6.1% vs. IORT: 32.0%±8.9%; Figure 2A). Also, no significant correlation between the volume of the WF and the plating efficiency was observed (R2No IORT=0.04, R2IORT=0.05; Figure 2B).
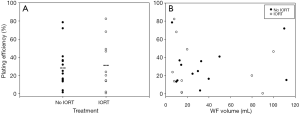
Discussion
In this work the effect of IORT on the proliferation and clonogenic growth capacity of WF obtained from breast cancer patients treated with or without IORT was investigated. Using 1% WF, a non-significant trend for an inhibiting effect of the IORT on the proliferative capacity was observed (Figure 1A) but not for 3% WF (P=0.16; Figure 1B). Similarly, no significant effect of the IORT on the clonogenic growth capacity of WF was observed either (Figure 2A). Our results from the short-term proliferation assay (MTT) with ER/PgR+ and Her2/neu− MCF7 cells complement previous data by Belletti et al. (8) on MDA-MB-231 (ER/PgR−-Her2/neu−), MDA-MB-453 and SKBR-3 (both ER/PgR−-Her2/neu+) and are broadly in line with the absence of a significant effect of IORT on WF-stimulated proliferation in 2-D cultures in these cell lines. Although stimulation of proliferation by WF was found to be higher in Her2/neu positive than in negative cell lines (6), IORT did not seem to have a significant effect on the proliferative capacity of WF irrespective of the estrogen, progesterone, and Her2 receptor status of the breast cancer cell line. However, it should be noted that in the present study MCF7 cells did not proliferate when supplemented with pure WF but required addition of 3% FBS in both assays. This is consistent with recent evidence from head and neck tumor cell lines that the stimulatory effect of WF on proliferation may be cell-line specific (14).
As WF volumes varied greatly, we speculated that this may potentially modulate the effects of the WF on the tested proliferation and clonogenic growth of MCF7 cells. The rationale behind this was that increased/decreased content of diluting liquid (blood/ lymph) will affect the concentration of growth modulating molecules in the WF. No correlation was detected between the WF volume and proliferation of MCF7 cells in either assay (Figure 1C,D and Figure 2B), thereby arguing against this hypothesis.
A limitation of the present study may be that WF did not stimulate proliferation of MCF7 cells as found previously for other breast cancer cell lines (6,8). However, the previous studies used WF in serum-free medium with peripheral blood serum as controls whereas MCF7 cells required 3% FBS for short-term and clonogenic proliferation. Furthermore, a significant reduction of WF-stimulated proliferation by IORT was not observed in short-term 2-D cultures of MDA-MB-231 (slight decrease; P=0.11), MDA-MB-453 (slight increase; P=0.2), or SKBR-3 (slight increase; P=0.1) in the study by Belletti et al. (8). Notably, they did not show data for MCF7 in 2-D culture but the non-significant decrease (P=0.07-0.16) observed for MCF7 in the present study together with the previous findings supports the conclusion that WF from IORT patients does not impair proliferation in 2-D culture. This was corroborated by the absence of an effect of IORT on WF-stimulated entry into the S-phase of the cell cycle (8).
It should be noted that the present results do not rule out that there may be differences in the cellular microenvironment and the cytokine composition of WF after IORT compared to no IORT. Thus, Belletti et al. (8) showed that IORT reduced WF-stimulated migration (chemotaxis assay) and invasion (Matrigel Transwell assay). This was associated with changes in the cytokine profile of WF by intraoperative tumor-bed irradiation as performed according to the TARGIT protocol (8). In addition, the size of MCF7 colonies grown in a Matrigel 3-D matrix was reduced. However, since 2-D area rather than cell numbers per colony or yield of colonies per cell seeded was measured, proliferation may have been confounded by invasion and migration in this assay.
In summary, the present study did not support an effect of WF from IORT patients on clonogenic or short-term proliferation of MCF7 breast cancer cells.
Acknowledgments
We would like to thank the staff of the Department of Radiation Oncology and the Department of Gynecology and Obstetrics (UMM, Mannheim) for their support.
Funding: The original study on IORT was funded by the German Federal Ministry of Education and Research (BMBF; grant FKZ01ZP0508).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Intraoperative Radiotherapy II”. The article has undergone external peer review.
Conflicts of Interest: Radiobiological research in our department is supported by Carl Zeiss Surgery, Oberkochen, Germany. FG, MS and FW receive speaker’s fees and honoraria by Carl Zeiss Meditec AG. For all other authors, there are no conflicts of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Medical Ethics Commission II of the Medical Faculty of Mannheim, Heidelberg University and was conducted according to Declaration of Helsinki principles. Informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cox TR, Erler JT. Molecular pathways: connecting fibrosis and solid tumor metastasis. Clin Cancer Res 2014;20:3637-43. [PubMed]
- Baum M, Demicheli R, Hrushesky W, et al. Does surgery unfavourably perturb the "natural history" of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer 2005;41:508-15. [PubMed]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738-46. [PubMed]
- Demicheli R, Valagussa P, Bonadonna G. Does surgery modify growth kinetics of breast cancer micrometastases? Br J Cancer 2001;85:490-2. [PubMed]
- Tsuchiya Y, Sawada S, Yoshioka I, et al. Increased surgical stress promotes tumor metastasis. Surgery 2003;133:547-55. [PubMed]
- Tagliabue E, Agresti R, Carcangiu ML, et al. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet 2003;362:527-33. [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Belletti B, Vaidya JS, D'Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008;14:1325-32. [PubMed]
- Herskind C, Wenz F. Radiobiological aspects of intraoperative tumour-bed irradiation with low-energy X-rays (LEX-IORT). Transl Cancer Res 2014;3:3-17.
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [PubMed]
- Sperk E, Welzel G, Keller A, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253-60. [PubMed]
- Neumaier C, Elena S, Grit W, et al. TARGIT-E(lderly)--prospective phase II study of intraoperative radiotherapy (IORT) in elderly patients with small breast cancer. BMC Cancer 2012;12:171. [PubMed]
- Liu Q, Schneider F, Ma L, et al. Relative Biologic Effectiveness (RBE) of 50 kV X-rays measured in a phantom for intraoperative tumor-bed irradiation. Int J Radiat Oncol Biol Phys 2013;85:1127-33. [PubMed]
- Ekblad L, Lindgren G, Persson E, et al. Cell-line-specific stimulation of tumor cell aggressiveness by wound healing factors - a central role for STAT3. BMC Cancer 2013;13:33. [PubMed]


