
Huge mesenteric fibromatosis presenting with intestinal perforation and acute diffuse peritonitis: a case report
Introduction
Mesenteric fibromatosis, also known as desmoid tumor is myofibroblastic proliferation of the mesentery. The fibromatosis comprises a broad group of myofibroblastic proliferations. It is classified into deep-seated and superficial, and the former is also called desmoid tumor (1,2). Desmoid tumor is usually divided into abdominal, extra-abdominal or intra-abdominal according to the anatomical location. The most occurring locations of desmoid tumor are the extremities, the abdominal wall, and intra-abdominal or mesenteric (3) (Table 1). Desmoid tumor accounted for 0.03% of all tumors, and less than 3% of all soft tissue tumors (4). Mesenteric fibromatosis accounts for about 8% of all cases of desmoid tumor. It often manifests as a bump in the abdomen, pain or discomfort. However, due to its relationship to important structures and its ability to penetrate adjacent organs, it may cause important complications, including intestinal obstruction, ischemia, perforation, fistula, hydronephrosis, and even aortic rupture or hilar obstructive (5-7). Here we report a huge mesenteric fibromatosis presenting with acute diffuse peritonitis, and this is the first report to treat an emergent case of huge mesenteric fibromatosis. We present the following case in accordance with the CARE Reporting Checklist (available at http://dx.doi.org/10.21037/tcr-19-1151).
Table 1
| Anatomic designation | Incidence (%) |
|---|---|
| Extra-abdominal | |
| Head and neck | 8 |
| Chest wall | 15 |
| Back | 6 |
| Upper extremity | 14 |
| Lower extremity | 16 |
| Pelvic girdle | 5 |
| Abdominal wall | 16 |
| Intra-abdominal | 25 |
Case presentation
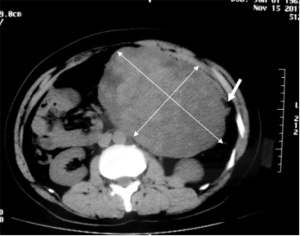
A 44-year-old Chinese man was admitted to our hospital because of an acute abdominal pain three hours ago. He complained a paroxysmal epigastric pain after breakfast, and then it became persistent and generalized followed by nausea. On admission, he had a fever of 38 °C. On physical examination, the whole abdomen was tender, especially in the upper quadrant. Further, a huge mass about 15 cm × 10 cm in size was palpated. He did not have any past history of emergent trauma or surgery. Laboratory test shown an increase in the percentage of neutrophils (94.6%, normal range, 50–70%) while the total white blood cell count was normal. Abdominal computed tomography (CT) scan showed pneumoperitoneum and ascites, suggesting there was hollow organ perforation. In addition, a homogeneous and hard mass sized 17 cm × 10 cm was found in the left abdomen (Figure 1).
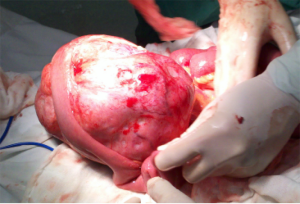
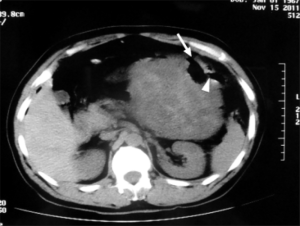
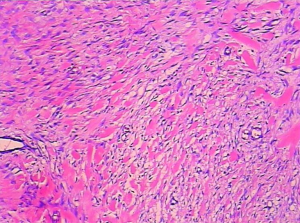
The patient underwent urgent laparotomy. A smooth and well-circumscribed lesion measuring about 17 cm × 10 cm × 10 cm was found at the mesenteric side of the jejunum (Figure 2). Further, there was a small perforation in the lesion and nearby jejunum, respectively (Figure 3). Initially, an intra-operative diagnosis of GIST was made. We resected the total lesion and partial jejunum. Histological examination showed many bundles of spindle-shaped cells (Figure 4). Immunohistochemistry showed that the tumor cells were negative for Bcl-2, CD117, CD34, CD99 and desmin, and partial positive for neuron-specific enolase (NSE), S-100 and smooth muscle actin (SMA), and strong positive for vimentin. However, the nuclear beta-catenin was negative. Based on the above results, the tumor was finally diagnosed as mesenteric fibromatosis. The post-operative course was uneventfully and the patient was discharged 2 weeks after surgery. With one-year follow-up, the patient had no recurrence (Figure 5).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Norther Jiangsu People’s Hospital Institutional Review Board. Informed written consent was obtained prior to study enrollment.
Discussion
Mesenteric fibromatosis can present as a sporadic case or as a part of familial adenomatous polyposis (FAP) or Gardener’s syndrome (8-10). Association with Crohn’s disease (CD) has been reported (11). Surgical experience, antecedent trauma, estrogen and pregnancy have been considered to increase the risk of mesenteric fibromatosis occurrence. The average age of patients with mesenteric fibromatosis is around 40. It is more common in females than males.
Mesenteric fibromatosis usually invades the mesentery of the small bowel. However, it can originate from retroperitoneum, omentum, ileocolic mesentery and gastrocolic ligament (12). It has a tendency to recur when it was excited incompletely or with Gardner’s syndrome, but rarely metastasize to other organs (13,14). Most of mesenteric fibromatosis present as insidious masses and sometimes infiltrate into adjacent structure asymptomatically (15). Approximately 16% of primary mesenteric fibromatosis were less than 5 cm, 28% were between 5 and 10 cm, and 50% were greater than 10 cm (16). Depending on its relationship to adjacent organs, serious complications might be caused, such as intestinal obstruction, perforation, ischemia, hydronephrosis, fistula, and even aortic rupture or infiltration of porta hepatis with obstructive jaundice (1-3).
Both MRI and CT are effective methods for pre-operative diagnosis. However, when the lesion involves the stomach or the intestine, it may appear to originate from these sites, and thereby mimic primary gastrointestinal tumor (GIST) (17). Generally mesenteric fibromatosis is easy to be confused with GIST (18). MRI has an advantage to monitor postoperative recurrence (19). In order to differentiate from Gardner’s syndrome, FAP or CD, colonoscopy is recommended.
Histology and IHC are useful methods to make definite diagnosis. The macroscopical appearance of desmoid tumor is a pale surface with poor vascularization (20). Microscopically spindle-shaped cells rich in cytoplasmic acidophilia were usually observed (21). IHC of desmoid tumor can be positive for the markers of vimentin, α-SMA, muscle actin, and desmin muscle cell. Besides loss-of-function mutations of APC, gain-of-function mutations of beta-catenin leading to nuclear beta-catenin immunoreactivity were also been described (22). IHC on hormone receptors show high positive rate of estrogen receptor (ER) beta receptor expression (89%) but no ER alpha (0%) (23).
In spite of total resection and R0 margin of mesenteric fibromatosis, a local relapse rate was up to 23–39% (24). In case of Garden’s syndrome, the relapse rate is higher than sporadic cases (13,25). Recurrent mesenteric fibromatosis can still be resected, but accompanied by increased morbidity and mortality (26). Further, radiotherapy is an optional choice for recurrent or advanced mesenteric fibromatosis (27). In addition, tyrosine kinase inhibitors showed a nice response in treating locally advanced mesenteric fibromatosis (28,29). The positive margin after surgery has a higher risk of recurrence. The risk of recurrence after incomplete surgical resection can be reduced by adjuvant radiotherapy (30). In cases of recurrent mesenteric fibromatosis, adjuvant radiation with a combined dose of ≥50 Gy showed a clear advantage over surgery alone (29).
In our case, we met an emergent case of huge mesenteric fibromatosis who complained a paroxysmal epigastric pain, and CT scan showed a huge mass in the left abdomen, as well as pneumoperitoneum and ascites. In an urgent laparotomy, we successfully resected the total lesion. Postoperative histology helped to differentiate it from GIST to mesenteric fibromatosis. In this emergent case, total resection and R0 margin is a key. However, due to its huge size, it was classified as high risk for recurrence, and a chemotherapy should be considered. Unfortunately, the patient rejected the chemotherapy. Although the patient had no recurrence one year after surgery, additional follow-up is needed.
In conclusion, mesenteric fibromatosis usually grows slowly and asymptomatically. Emergent conditions (i.e., hollow organ perforation and acute diffuse peritonitis) are not common. CT and MRI are the preferred methods for diagnosis. GIST remains the most important differential diagnosis, especially when it involves the GI tract. Histologic and IHC examinations, i.e., beta-catenin, APC gene and hormone receptors, are useful to make a definite diagnosis. Surgical resection remains the first choice to treat mesenteric fibromatosis (28).
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE Reporting Checklist. Available at http://dx.doi.org/10.21037/tcr-19-1151
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-1151). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Norther Jiangsu People’s Hospital Institutional Review Board. Informed written consent was obtained prior to study enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stout AP. Fibrosarcoma the malignant tumor of fibroblasts. Cancer 1948;1:30-63. [Crossref] [PubMed]
- Molnar PP. Enzinger and Weiss's Soft Tissue Tumors (ed 4). Hum Pathol 2002;32:1414.
- Lev D, Kotilingam D, Wei C, et al. Optimizing Treatment of Desmoid Tumors. J Clin Oncol 2007;25:1785. [Crossref] [PubMed]
- Micke O, Seegenschmiedt MH. Radiation therapy for aggressive fibromatosis (desmoid tumors): results of a national Patterns of Care Study. Int J Radiat Oncol Biol Phys 2005;61:882-91. [Crossref] [PubMed]
- Collins D, Myers E, Kavanagh D, et al. Mesenteric desmoid tumor causing ureteric obstruction. Int J Urol 2008;15:261-2. [Crossref] [PubMed]
- Karagulle E, Gokturk HS, Turk E, et al. Intestinal perforation from primary intra-abdominal fibromatosis. Saudi Med J 2007;28:639-40. [PubMed]
- Holubar S, Dwivedi AJ, O'Connor J. Giant mesenteric fibromatosis presenting as small bowel obstruction. Am Surg 2006;72:427-9. [Crossref] [PubMed]
- Forte MD, Brant WE. Spontaneous isolated mesenteric fibromatosis. Report of a case. Dis Colon Rectum 1988;31:315-7. [Crossref] [PubMed]
- Al-Nafussi A, Wong NACS. Intra-abdominal spindle cell lesions: a review and practical aids to diagnosis. Histopathology 2001;38:387-402. [Crossref] [PubMed]
- Dong-Heup K, Kim DH, Goldsmith HS, et al. Intra-abdominal desmoid tumor. Cancer 1971;27:1041-5. [Crossref] [PubMed]
- Slater G, Greenstein AJ. Mesenteric fibromatosis in Crohn's disease. J Clin Gastroenterol 1996;22:147. [Crossref] [PubMed]
- Weiss SW, Goldblum JR. Malignant tumors of peripheral nerves. In: Weiss SW, Goldblum JR. editor. Enzinger and Weiss's Soft Tissue Tumors, 2008.
- Burke AP, Sobin LH, Shekitka KM, et al. Intra-abdominal fibromatosis. A pathologic analysis of 130 tumors with comparison of clinical subgroups. Am J Surg Pathol 1990;14:335. [Crossref] [PubMed]
- Yannopoulos K, Stout AP. Primary solid tumors of the mesentery. Cancer 1963;16:914-27. [Crossref] [PubMed]
- Easter DW, Halasz NA. Recent trends in the management of desmoid tumors. Summary of 19 cases and review of the literature. Ann Surg 1989;210:765-9. [Crossref] [PubMed]
- de Camargo VP, Keohan ML, D'Adamo DR, et al. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 2010;116:2258-65. [PubMed]
- Yantiss RK, Spiro IJ, Compton CC, et al. Gastrointestinal stromal tumor versus intra-abdominal fibromatosis of the bowel wall: a clinically important differential diagnosis. Am J Surg Pathol 2000;24:947-57. [Crossref] [PubMed]
- Rosai J. GIST: an update. Int J Surg Pathol 2003;11:177-86. [Crossref] [PubMed]
- Murayama T, Imoto S, Ito M, et al. Mesenteric fibromatosis presenting as fever of unknown origin. Am J Gastroenterol 1992;87:1503-5. [PubMed]
- Cotran RS. Diseases of immunity. Robbins Pathologic Basis of Disease, 1999.
- Salloum H, Kanitakis J, Chouvet B, et al. Extra-abdominal desmoid tumor. Microscopic aspects and histogenesis. Ann Dermatol Venereol 1993;120:685-8. [PubMed]
- Li C, Bapat B, Alman BA. Adenomatous polyposis coli gene mutation alters proliferation through its beta-catenin-regulatory function in aggressive fibromatosis (desmoid tumor). Am J Pathol 1998;153:709-14. [Crossref] [PubMed]
- Santos GA, Cunha IW, Rocha RM, et al. Evaluation of estrogen receptor alpha, estrogen receptor beta, progesterone receptor, and cKIT expression in desmoids tumors and their role in determining treatment options. Biosci Trends 2010;4:25-30. [PubMed]
- Karakousis CP, Mayordomo J, Zografos GC, et al. Desmoid tumors of the trunk and extremity. Cancer 1993;72:1637-41. [Crossref] [PubMed]
- Al Jadaan SA, Al Rabeeah A. Mesenteric fibromatosis: case report and literature review. J Pediatr Surg 1999;34:1130-2. [Crossref] [PubMed]
- Rampone B, Pedrazzani C, Marrelli D, et al. Updates on abdominal desmoid tumors. World J Gastroenterol 2007;13:5985-8. [Crossref] [PubMed]
- Suit H, Spiro I. Radiation in the multidisciplinary management of desmoid tumors. Front Radiat Ther Oncol 2001;35:107-19. [Crossref] [PubMed]
- Kasper B, Baumgarten C, Bonvalot S, et al. Management of sporadic desmoid-type fibromatosis: A European consensus approach based on patients’ and professionals’ expertise – A Sarcoma Patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma G. Eur J Cancer 2015;51:127-36. [Crossref] [PubMed]
- Seinen JM, Niebling MG, Bastiaannet E, et al. Four different treatment strategies in aggressive fibromatosis: A systematic review. Clin Transl Radiat Oncol 2018;12:1-7. [Crossref] [PubMed]
- Janssen ML, van Broekhoven DL, Cates JM, et al. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg 2017;104:347. [Crossref] [PubMed]



