PD-1 inhibitor combined with apatinib for advanced gastric or esophagogastric junction cancer: a retrospective study
Introduction
Gastric or esophagogastric junction cancer (GC/EGJC) is the third leading cause of cancer-related deaths worldwide, with most of the cases diagnosed at late stages (1,2). For patients (pts) with unresectable or recurrent advanced GC/EGJC, systemic chemotherapy is the most important method used to prolong survival (3). However, research data indicate that the objective response rate (ORR) ranges from 6.8–25% and the progression-free survival (PFS) was 1.5–5.3 months in second or further lines therapy (4-6), showing a poor prognosis. With this background, new clinical treatment approaches for advanced GC/EGJC are urgently needed, particularly in later lines. Immune checkpoint inhibitors have been shown to be effective in treating GC through blocking the interaction between programmed cell death-1 (PD-1) and its ligand (PD-L1) (7,8). Of these, nivolumab and pembrolizumab, were approved as third-line therapies for advanced GC/EGJC in 2017. However, only about 10% of advanced GC/EGJC patients benefit from monotherapy overall (9,10). Therefore, to extend the benefit to a larger population, the development of innovative strategies such as combining PD-1/PD-L1 blockade with conventional treatments is urgently needed in advanced GC/EGJC.
In recent years, studies have shown that immunotherapy has a synergistic effect when combined with molecular antiangiogenic agents (11,12). Anti-angiogenesis is a well-established tumor microenvironment (TME) targeted therapy in GC/EGJC. Combining PD-1/PD-L1 blockade with agents that can eliminate the preexisting immunosuppression of TME may overcome the primary resistance in patients with advanced GC/EGJC (13-15). Moreover, in preliminary results from an open-label clinical trial, with a combination of anti-PD-1 antibody and VEGFR1-3 inhibitor, nivolumab plus regorafenib achieved an ORR of 44% (5/9) in pts with pretreated GC (16), which provides rationale to apply immunotherapy combined with molecular antiangiogenic agents for GC/EGJC.
Apatinib, a small-molecule anti-angiogenesis targeted drug, has been approved as third-line or above therapy for pts with advanced GC/EGJC in China (17). In in vitro studies, apatinib and PD-1 inhibitor have shown complementary anti-tumor effects (18,19). Based on these results, we carried out a retrospective clinical research study to assess the value of clinical application of PD-1 inhibitor and apatinib as combination therapy in pts with advanced GC/EGJC. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1333).
Methods
Study population
Our study collected 24 pts with histologically confirmed, unresectable locally advanced or metastatic HER2-negative GC/EGJC treated with PD-1 inhibitor combined with apatinib in Zhejiang Cancer Hospital from May 2018 to May 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhejiang Cancer Hospital (IRB-2019-155) and written informed consent was obtained from all patients. The Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 was selected. Other inclusion criteria included PD-1 inhibitor combined with apatinib for more than two cycles, and at least one target lesion that could be measured by imaging. Pts with autoimmune diseases and who had received any PD-1, PD-L1, or other drug immunotherapy were excluded. Further details are shown in Table 1.
Table 1
| Characteristic | No (%) (n=24) |
|---|---|
| Age, years, median [range] | 60.5 [30–74] |
| Gender | |
| Male | 18 (75.0) |
| Female | 6 (25.0) |
| ECOG | |
| 0 | 4 (16.7) |
| 1 | 16 (66.6) |
| 2 | 4 (16.7) |
| Histology subtype (Lauren classification) | |
| Intestinal | 5 (20.8) |
| Diffuse | 10 (41.7) |
| Mixed | 4 (16.7) |
| Unknown | 5 (20.8) |
| Number of metastatic sites | |
| 1–2 | 9 (37.5) |
| ≥3 | 15 (62.5) |
| Peritoneal metastases | |
| Yes | 17 (70.8) |
| No | 7 (29.2) |
| Liver metastases | |
| Yes | 13 (54.2) |
| No | 11 (45.8) |
| Prior therapies | |
| Surgery | 14 (58.3) |
| 1st line therapy | 16 (66.6) |
| >1st line therapy | 8 (33.4) |
| Immunotherapy drugs | |
| JS001 | 15 (62.5) |
| Sintilimab | 6 (25.0) |
| Nivolumab | 1 (4.2) |
| SHR-1210 | 2 (8.3) |
ECOG, Eastern Cooperative Oncology Group.
Study design and assessments
Our study is a retrospective, single-center study. Pts received apatinib at doses of 250 mg in combination with PD-1 inhibitor including SHR-1210 (200 mg Q2W), nivolumab (3 mg/kg Q2W), JS001 (240 mg Q3W), or sintilimab (200 mg Q3W). Treatment was continued until disease progression, intolerable toxicity, or other reason for termination was judged by the investigator.
The efficacy and safety of PD-1 inhibitor plus apatinib in advanced GC/EGJC pts are the primary objectives to be evaluated. Tumor assessments were performed through CT or MRI after every two cycles of treatment according to the RECIST v1.1 guideline. Observed indicators included ORRs, disease control rates (DCRs), PFS, and overall survival (OS). Adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events (version 4.0). If any grade ≥3 AEs occurred, apatinib or PD-1 inhibitor were discontinued until the adverse reaction returned to ≤1 degree. If the adverse reactions caused by apatinib lead to treatment delays of more than 4 weeks, apatinib was discontinued.
Statistical analysis
The distributions of PFS and OS were estimated using the Kaplan-Meier (KM) method. The statistical significance of survival curves was tested with a log-rank test. All data were analyzed by SPSS 20 statistical analysis.
Results
Patient information and baseline characteristics
Twenty-four pts were enrolled to receive SHR-1210 (n=2), nivolumab (n=1), JS001 (n=15), or sintilimab (n=6), until the data cutoff (December 31, 2019). The median age was 60.5 (range, 30–74) years and 75% (18/24) were male. Fifteen (62.5%) pts had multiple metastatic lesions (≥3) and 58.3% (14/24) pts had undergone surgery. Of the 24 pts, 16 (66.6%) and 8 (33.4%) pts had previously received first-line treatment and more than first-line treatment, respectively. Additional details are provided in Table 1.
Efficacy
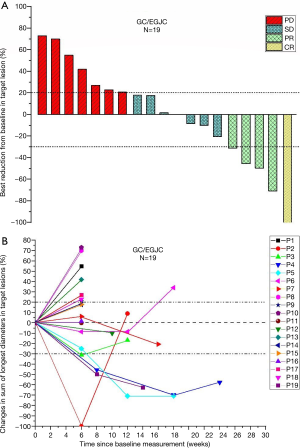
Of 24 pts, 19 pts were evaluable by RECIST v1.1. One patient achieved complete response (CR), four pts achieved partial response (PR), seven pts achieved stable disease (SD), and seven pts had progressive disease (PD). The ORR was 26.3% (5/19), and the DCR was 63.2% (12/19).
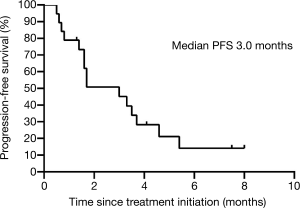
Median time to response was 1.7 (interquartile range, 1.6 to 2.1) months. Median duration of response was 3.0 (interquartile range, 1.8 to 3.7) months. The median PFS was 3.0 (95% CI, 1.3 to 4.7) months (Figure 1, Table 2). The median OS was not reached (Table 2).
Table 2
| Evaluation | Value |
|---|---|
| RECIST v1.1 tumor evaluation | |
| CR | 1 |
| PR | 4 |
| SD ≥6 weeks | 7 |
| PD | 7 |
| Not evaluable | 5 |
| ORR in evaluable patients | 26.30% |
| DCR in evaluable patients | 63.20% |
| Median time to response | 1.7 months |
| Duration of response | |
| KM median | 3.0 months |
| PFS | |
| KM median | 3.0 months |
| OS | |
| KM median | NR |
GC, gastric cancer; EGJC, esophagogastric junction cancer; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate; KM, Kaplan-Meier; PFS, progression-free survival; OS, overall survival; NR, not reached.
Specifically to explain the efficacy and changes in tumor regression, we show the best percentage change in lesion size of the 19 pts in our study in Figure 2A and percentage changes over time in Figure 2B.
Safety
Safety analysis of this study in 19 pts is as follows. Treatment-related adverse events (TRAEs) leading to discontinuation were reported in 3 (15.8%) of the 19 pts. These included liver function damage [increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], rash and pruritus, and hand-foot syndrome. All-grade TRAEs reported in ≥5% of patients are summarized in Table 3. Relatively few grade 3 or 4 TRAEs occurred, but these included pruritus (5.3%), rash (5.3%), hand-foot syndrome (5.3%), and increased AST (5.3%) and ALT (5.3%). No treatment-related deaths occurred.
Table 3
| Total (n=19), n (%) | ||
|---|---|---|
| Any grade | Grade 3/4 | |
| TRAEs | 3 (15.8) | 3 (15.8) |
| Common TRAE | ||
| Decreased appetite | 5 (26.3) | 0 |
| Diarrhea | 3 (15.8) | 0 |
| Nausea | 3 (15.8) | 0 |
| Fatigue | 6 (31.6) | 0 |
| Vomiting | 4 (21.1) | 0 |
| Abdominal pain | 3 (15.8) | 0 |
| Pyrexia | 1 (5.3) | 0 |
| Pruritus | 2 (10.5) | 1 (5.3) |
| Rash | 2 (10.5) | 1 (5.3) |
| Hand-foot syndrome | 4 (21.1) | 1 (5.3) |
| Proteinuria | 1 (5.3) | 0 |
| AST increase | 7 (36.8) | 1 (5.3) |
| Blood bilirubin increase | 6 (31.6) | 0 |
| ALT increase | 7 (36.8) | 1 (5.3) |
| Hematological AE | ||
| Platelet count decrease | 5 (26.3) | 0 |
| Leukopenia decrease | 4 (21.1) | 0 |
| Neutropenia decrease | 4 (21.1) | 0 |
| Hemoglobin decrease | 3 (15.8) | 0 |
| Additional TRAEs of special interest | ||
| Interstitial lung disease | 0 | 0 |
| Colitis | 0 | 0 |
| Hypopituitarism | 0 | 0 |
| Thyroid disorder | 8 (42.1) | 0 |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
In this retrospective study, the ORR following PD-1 inhibitor and apatinib combination therapy was 26.3%, and the PFS was 3.0 months. Compared with previous study results, in which that ORR ranges from 6.8–25% and the PFS was 1.5–5.3 months (4-6), a significant increase of ORR was shown. Simultaneously, DCR was observed in 63.2% (12/19) of pts in our study, with median duration of response of 3.0 (interquartile range, 1.8 to 3.7) months. Long-lasting responses existed. In a study of apatinib monotherapy for advanced GC/EGJC in third-line and above treatment, the median PFS was 2.6 months, ORR was 2.84%, and DCR was 42.05% (20). Our results show that the efficacy of PD-1 inhibitor and apatinib combination therapy turned out to be better compared to apatinib monotherapy.
Nivolumab and pembrolizumab have been approved as immune checkpoint inhibitors for third-line treatment indications for advanced GC/EGJC. But till now, the ORR of immunotherapy monotherapy is only 11–23% (21-23), which emphasizes the necessity of changing treatment options to improve efficacy. Results of this research show that PD-1 inhibitor and apatinib combination therapy improve the efficacy of treatment, mainly because first, tumor angiogenesis inhibits the extravasation of reactive T cells, which form an immunosuppressive microenvironment that leads to tumors escaping immunosurveillance. Combination therapy strengthens T-cell infiltration and activation to eliminate tumor cells (24-27). In addition, Jain et al. and Huang et al. demonstrated that anti-angiogenic therapy causes vascular normalization, mitigating hypoxia, and may allow more effective T cells to extravasate from the blood into the TME and enhance cancer immunotherapies (28,29). Moreover, anti-vascular targeted therapy apatinib may enhance anti-tumor immune responses by breaking oncogene dependence, which, in turn, causes cancer cell senescence and promotes T-cell clearance (30). Zhao and his team found that low-dose apatinib (250 mg/d) could impede the recruitment of tumor-associated macrophages, decrease TGF-β level, block tumor growth and metastasis, and eventually cause prolonged survival in mouse and in vitro models (31).
The safety profile of combination therapy in pts with advanced GC/EGJC was manageable. Adverse reactions are controllable. The types of adverse reactions were consistent with those known to be related to PD-1 inhibitor and apatinib (7,9,20,23). Thyroid disorders are associated with PD-1 inhibitor. All of the adverse effects we found were grades 1 and 2. This may be related to the possibility that PD-1 inhibitors may modulate the immune balance and stimulate their own immune potential (32). We found that hand-foot syndrome, proteinuria, decreased platelet count, leukopenia, and neutropenia were associated with apatinib. TRAEs leading to discontinuation were reported in three pts. These three pts had to discontinue treatment due to liver function damage (AST and ALT increases), rash and pruritus, and hand-foot syndrome. It seems that combination therapy leads to a slight increase in adverse reactions, including ALT and AST increases. No treatment-related deaths occurred. The combination therapy is safe and reliable in clinical application.
There were some limitations in our research. First, this report was a single-center retrospective study with insufficient sample and possibly incomplete information, resulting in recall bias. Additionally, we lacked data, including biomarkers PD-L1 and tumor mutation burden, which would have been related to the efficacy of immunological checkpoint inhibitors. We will explore this further in the future.
In conclusion, PD-1 inhibitor and apatinib combination therapy has shown encouraging clinical activity, can improve survival, and demonstrates tolerable toxicity in pts with advanced GC/EGJC as second- or third-line therapy. We expect further research, especially in the field of first-line or neoadjuvant therapy, to continue exploring the value of this combination therapy in advanced GC/ EGJC.
Acknowledgments
We thank all researchers and patients for participating in this trial.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1333
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1333
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1333). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhejiang Cancer Hospital (IRB-2019-155) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Catalano V, Labianca R, Beretta GD, et al. Gastric cancer. Crit Rev Oncol Hematol 2009;71:127-64. [Crossref] [PubMed]
- Niccolai E, Taddei A, Prisco D. Gastric cancer and the epoch of immunotherapy approaches. World J Gastroenterol 2015;21:5778-93. [Crossref] [PubMed]
- Chan WL, Yuen KK, Siu SW, et al. Third-line systemic treatment versus best supportive care for advanced/metastatic gastric cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:68-81. [Crossref] [PubMed]
- Galdy S, Cella CA, Spada F, et al. Systemic therapy beyond first-line in advanced gastric cancer: an overview of the main randomized clinical trials. Crit Rev Oncol Hematol 2016;99:1-12. [Crossref] [PubMed]
- Takahari D. Second-line chemotherapy for patients with advanced gastric cancer. Gastric Cancer 2017;20:395-406. [Crossref] [PubMed]
- Magalhães H, Fontes-Sousa M, Machado M. Immunotherapy in advanced gastric cancer: an overview of the emerging strategies. Can J Gastroenterol Hepatol 2018;2018:2732408. [Crossref] [PubMed]
- Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol 2014;20:1657-66. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Fashoyin-Aje L, Donoghue M, Chen H, et al. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist 2019;24:103-9. [Crossref] [PubMed]
- Manegold C, Dingemans AC, Gray JE, et al. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol 2017;12:194-207. [Crossref] [PubMed]
- Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013;39:74-88. [Crossref] [PubMed]
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237-51. [Crossref] [PubMed]
- Hamzah J, Jugold M, Kiessling F, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 2008;453:410-4. [Crossref] [PubMed]
- Tartour E, Pere H, Maillere B, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 2011;30:83-95. [Crossref] [PubMed]
- Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol 2020;38:2053-61. [Crossref] [PubMed]
- Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Letters 2016;372:187-91. [Crossref] [PubMed]
- Schoenfeld JD, Dranoff G. Anti-angiogenesis immunotherapy. Hum Vaccin 2011;7:976-81. [Crossref] [PubMed]
- Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 2013;172:500-6. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Coutzac C, Pernot S, Chaput N. Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol 2019;133:25-32. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncology 2018;4:e180013. [Crossref] [PubMed]
- Huang J, Mo H, Zhang W, et al. Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer 2019;125:742-9. [Crossref] [PubMed]
- Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity 2013;39:61-73. [Crossref] [PubMed]
- Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017;9:eaak9679. [Crossref] [PubMed]
- Lanitis E, Irving M. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 2015;33:55-63. [Crossref] [PubMed]
- Tang H, Wang Y, Chlewicki L, et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 2016;29:285-96. [Crossref] [PubMed]
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nature Med 2001;7:987-9. [Crossref] [PubMed]
- Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A 2012;109:17561-6. [Crossref] [PubMed]
- Rakhra K, Bachireddy P, Zabuawala T, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 2010;18:485-98. [Crossref] [PubMed]
- Zhao S, Ren S, Jiang T, et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res 2019;7:630-43. [Crossref] [PubMed]
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583-9. [Crossref] [PubMed]