
Expression of combined interference of slug and FoxC2 in endometrial carcinoma and its clinicopathological relationship
Introduction
Endometrial carcinoma is one of the most malignant tumors in female reproductive system, which has severely damaged the life health in women. However, there is still lacking effective prognostic indicators and treatment options for the clinical treatments. Slug is a transcription factor of SNAIL family that can encode the zinc finger protein, which is reported to be up-regulated in multiple malignant tumors. FoxC2 is a member of the Fox protein family, which can regulate the expression of a series of downstream genes, and participate in the epithelial-mesenchymal transition (EMT) (1-3). Transcription factors Slug and FoxC2 have been reported to play important roles in tumor invasion and metastasis, which are the important regulatory molecules during tumor cell migration and metastasis (4).
Short hairpin RNA (shRNA) is a kind of RNA sequence that can form a tight hairpin turn, which can silence gene expression. The double-strand small molecule RNA or shRNA has been utilized in clinical test to treat several diseases, and certain achievements have been attained in terms of tumor treatment. In the current study, shRNAs were used to down-regulate the Slug and FoxC2 expression in Ishikawa and RL-952 endometrial carcinoma cell lines, and cell proliferation, migration and invasion were further evaluated. Then we tested the relevant indicators of EMT after transfection to gain a deeper understanding of the role of Slug and FoxC2 in endometrial carcinoma.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/tcr-20-809).
Methods
Patients
The endometrial carcinoma tissues were retrospectively collected from 124 patients in the Pathology Department of the First Affiliated Hospital of Bengbu Medical College from January 2014 to December 2015. Before surgery, all endometrial carcinoma patients did not receive any anticancer treatments, including chemotherapy and radiotherapy. Patients with endometrial carcinoma were confirmed by pathologic diagnosis. The normal endometrial tissues were collected into the control group, which were derived from 35 patients receiving endometrial tissue biopsy and hysterectomy due to other benign disease in Gynecology.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of The First Affiliated Hospital of Bengbu Medical College (No. BBMEC-2018-10) and informed consent was taken from all the patients.
Immunohistochemical staining
Endometrial tissues resected from uterus of patients, were rinsed with normal saline, fixed with 4% formalin for 24 h, dehydrated and embedded. The samples were sliced into sections 0.4 µm in thickness, followed by dewaxing of the sections and antigen retrieval in citric acid solution for 3 min at 121 °C. After natural cooling, the sections were treated with 3% H2O2 solution for 10 min, followed by the addition of anti-Slug (ab180714, Abcam, Cambridge, MA, USA) and anti-FoxC2 (ab5060, Abcam, Cambridge, MA, USA) rabbit anti-human primary antibodies to incubate for 1 h at 60 °C, rinsing with PBS for 3×5 min and incubation with secondary antibody for 30 min at 37 °C. Then, the sections were washed with PBS, followed by DAB staining for visualization, nuclear counterstaining of hematoxylin and microscopic observation. The Image-Pro Plus software was adopted for image analysis by another person who was blind to the group assignments, and the integrated optical density/area ratio was used as the criterion to determine the protein expression contents of Slug and FoxC2.
Cell culture and transfection
Human endometrial carcinoma cell lines Ishikawa (catalog number BH-X5152, Shanghai Bohu Biotechnology Co., Ltd.) and RL-952 (catalog number BNN1364, Shanghai Beinuo Biology Co., Ltd.) were maintained in RPMI 1640 medium (HyClone, Thermo Scientific) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin, and incubated in a humidified incubator under the conditions of 5% CO2 and 95% air at 37 °C. Subsequently, Ishikawa and RL-952 cells at logarithmic growth phase were collected and seeded randomly into a 96-well plate (2×104 cells/mL) and cultured for 24 h. The 2–5 generations cells were used for the following experiments.
The target plasmid DNA and Lipofectamine 2000 were diluted in the serum-free Opti-MEM. Cells at the density of 60–70% were collected and incubated with the mixed medium for 20 min at room temperature. Then, the mixtures were added to the 96-well plate containing both cells and culture solution, agitated gently, and placed in the incubator. The Slug-shRNA and FoxC2-shRNA expression vector (GenePharma, Shanghai, China) treatment groups (containing cells, complete medium, Slug-shRNA and FoxC2-shRNA), negative control group (containing cells, complete medium, and negative control-shRNA), and blank control group (containing cells and complete medium) were set, and 4 duplicates were prepared for each group.
Protein expression changes detected by Western blotting
Cells were harvested at 48 h after transfection, and lysed with the cell lysis buffer. Equivalent amounts of the sample lysate were then separated through sodium dodecyl sulfate polyacrylamide gel electrophoresis, which were later transferred onto the nitrocellulose membrane (Millipore) by electroblotting. Later, the membrane was blocked with 5% non-fat milk in the TBST buffer (containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl and 0.1% Tween 20) at 4 °C overnight, followed by incubation with specific primary antibodies for 2 h and the specific IgG HRP-conjugated secondary antibody for 1 h at room temperature. Details for the primary antibodies were as follows: Slug (1:2,000, catalog number # ab180714) and FoxC2 (1:2,000, catalog number #ab5060) were obtained from Abcam (Cambridge, MA, USA), β-actin (1:1,000, catalog number #bs-10021) was purchased from Bioworld (CA, USA). The resultant signals would then be visualized through enhanced chemiluminescence (ECL) (Pierce) on the Syngene G: BOX Chemi gel documentation system (Syngene, Cambridge, UK). The densitometric values would be normalized in each group, with β-actin being used as the internal reference.
Real-time quantitative PCR analysis
Cells in each group were harvested at 48 h after transfection, and the total RNA would then be isolated using the TRIzol kit (Invitrogen, CA, USA) in accordance with the manufacturer’s instructions. 1 µg total RNA was used to synthesize cDNA using a PrimeScript reagent Kit with DNAEraser (Takara Bio, Kyoto, Japan), followed by RT-PCR analysis using the SYBR green Master Mix (Takara Bio, Kyoto, Japan). The following primer pairs were obtained from Invitrogen (NY, USA), while GAPDH served as the internal reference.
GAPDH: 5'-GAAGAGTCAAGAGCAGTCGTCAAGA-3' (forward), 5'-GTAAGACCGAACCACAGTCAAGAGAG-3' (reverse); Slug: 5'-GAGCCTTCAAGAGAAAAAAGGAATT-3' (forward), 5'-GTAGTCAAGAGTCTTTTTTCCTTAA-3' (reverse); FoxC2: 5'-GAGCCTTCAAGAGCCAGGTGGAATT-3' (forward), 5'-GTTCCGTCTTCTCAAGAGACGAATA-3' (reverse).
CCK-8 colorimetry assay
Ten µL CCK-8 was added into each well at 24, 48, 72 and 96 h, respectively, and further cultured for 4 h, and the optical density value in each well was measured using a microplate reader. Subsequently, the growth curve was plotted, and the average of 4 wells in each group was used to calculate the cell proliferation capacity. Each experiment was repeated for three times.
Transwell chamber migration assay
Cells in each group were digested with trypsin and washed with the serum-free culture medium. Cell suspension was prepared, and 200 µL was evenly added into the upper Transwell chamber (Beyotime Biotechnology, Haimen, China), while 600 µL complete medium was added to the lower Transwell chamber for 48 h. Then, the chambers were taken out, washed with PBS, fixed with 4% paraformaldehyde for 20 min, and stained with 1% crystal violet staining solution for 10 min. Subsequently, cells on the basilar membrane surface were wiped using the cotton swab, and the number of cells penetrating the membrane was counted under microscope. Each experiment was repeated for three times.
Transwell invasion assay
Before Transwell invasion assay, cells were deprived of serum for 24 h, resuspended (5×104) with 200 µL serum-free medium after trypsin digestion, and added onto the Matrigel surface in the upper Transwell chamber, while 600 µL complete medium was added to the lower Transwell chamber as the chemotaxin. The number of cells penetrating the Matrigel was counted under microscope. Each experiment was repeated for three times.
ELISA assay
The concentrations of MMP-2 and MMP-9 were further evaluated in both cell lines. Cell culture supernatants were collected at 48 h after transfection. MMP-2 and MMP-9 concentrations were measured using human MMP-2 ELISA Kit and human MMP-9 ELISA Kit (Invitrogen Corporation, Carlsbad, United States) according to the manufacturer’s instructions. The optical density (OD) value at 450 nm was record using a microplate reader.
Statistical analysis
Statistical analyses were performed using SPSS 18 for Windows (SPSS Inc., Chicago, IL, USA). Data are given as means ± SD and One-way analysis of variance followed by post-hoc Bonferroni analysis of variance. The two groups were compared with Student’s t-test. Each experiment was repeated for three times. P<0.05 was taken as a significant difference between groups.
Results
Slug and FoxC2 were highly expressed in endometrial carcinoma tissues
As shown in Figure 1, immunohistochemical results indicated positive expression in the nuclei of endometrial carcinoma tissues. Compared with normal endometrial tissues, the Slug and FoxC2 expression levels in endometrial carcinoma tissues were remarkably increased. The positive rate of Slug and FoxC2 in endometrial carcinoma was 61.3% (76/124) and 71.0% (88/124), and the difference was statistically significant compared with that in normal endometrial tissues (Table 1).
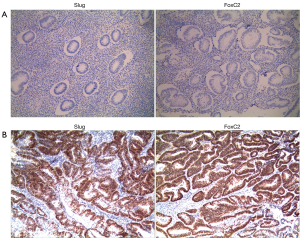
Table 1
| Histological type | Cases | Slug | Fox | |||||
|---|---|---|---|---|---|---|---|---|
| (–) | (+) | P value | (–) | (+) | P value | |||
| Normal endometrial tissues | 35 | 27 | 8 | <0.01 | 31 | 4 | <0.01 | |
| Endometrial carcinoma tissues | 124 | 48 | 76 | 36 | 88 | |||
P<0.01 vs. normal and atypical hyperplastic group.
Relationships of Slug and FoxC2 expression with the clinicopathological factors of endometrial carcinoma
There was no relationship between the Slug and FoxC2 expression and age in each group (P>0.05). Slug expression in endometrial carcinoma was notably correlated with the histological grade, muscular layer infiltration and lymph node metastasis (P<0.05), but it was not markedly correlated with The International Federation of Gynecology and Obstetrics (FIGO) stage. FoxC2 expression was distinctly related to the FIGO stage and lymph node metastasis (P<0.05), but it was not correlated with histological grade and muscular layer infiltration (Table 2).
Table 2
| Factors | Cases | Slug | FoxC2 | |||||
|---|---|---|---|---|---|---|---|---|
| (–) | (+) | P | (–) | (+) | P | |||
| Age | ||||||||
| <50 | 29 | 10 | 19 | 16 | 13 | |||
| ≥50 | 95 | 38 | 57 | 0.593 | 50 | 45 | 0.810 | |
| Histological grade | ||||||||
| G1 | 57 | 29 | 28 | 33 | 24 | |||
| G2 | 41 | 14 | 27 | 20 | 21 | |||
| G3 | 26 | 5 | 21 | 0.018* | 13 | 13 | 0.627 | |
| FIGO stage | ||||||||
| I + II | 101 | 42 | 59 | 59 | 42 | |||
| III + IV | 23 | 6 | 17 | 0.168 | 7 | 16 | 0.015# | |
| Muscular layer infiltration | ||||||||
| ≤1/2 | 84 | 39 | 45 | 49 | 35 | |||
| >1/2 | 40 | 9 | 31 | 0.011& | 17 | 23 | 0.099 | |
| Lymph node metastasis | ||||||||
| No | 93 | 41 | 52 | 55 | 38 | |||
| Yes | 31 | 7 | 24 | 0.033$ | 11 | 20 | 0.022$ | |
*, P<0.05 vs. G1 and G2; #, P<0.05 vs. I + II stage; &, P<0.05 vs. invasion ≤1/2 group; $, P<0.05 vs. absent group.
Slug and FoxC2 protein expression were detected by Western blotting and RT-PCR
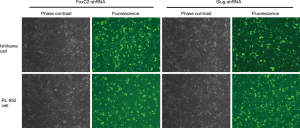
Ishikawa and RL-952 cells were first observed at 48h after shRNAs transfection (Figure 2). The fluorescence images indicated that the cells were successfully transfected with the shRNAs. Western blotting and RT-PCR results also indicated that, compared with control group, Slug and FoxC2 protein and mRNA levels in Ishikawa (Figure 3) and RL-952 (Figure 4) cells were remarkably reduced after Slug-shRNA and FoxC2-shRNA interference (P<0.01). Non-specific shRNA group showed no significant difference compared with those in blank control group (P>0.05).
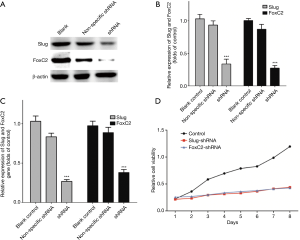
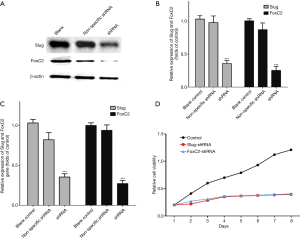
Slug-shRNA and FoxC2-shRNA inhibited endometrial carcinoma cell proliferation
As shown in Figure 3D and Figure 4D, the proliferation rates of both cell lines in the control group were markedly higher than those in shRNAs treatment groups. Independent sample t-test results suggested that, the differences in the proliferation capacity of cells in expression vector treatment groups were statistically significant compared with that in the other two groups at days 2, 3, 4, 5, 6 and 7 of growth (P<0.05). CCK-8 assay suggested that, the proliferation capacity of cells in shRNAs treatment groups was apparently lower than that in the control group.
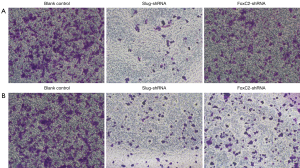
Slug-shRNA and FoxC2-shRNA inhibited endometrial carcinoma cell migration and invasion
The migration and invasion abilities in Ishikawa and RL-952 cells were then evaluated. The numbers of Ishikawa cells penetrating the filter membrane into the lower chamber in Slug-shRNA and FoxC2-shRNA groups were 174.58±8.11 and 165.33±7.65, respectively. The numbers of cells penetrating Matrigel into the lower chamber in Slug-shRNA and FoxC2-shRNA groups were 94.02±6.57 and 84.56±8.36, respectively. While those in the control group and blank control group were 261.76±10.11 in migration assay and 175.02±9.65 in invasion assay (Figure 5). The results in RL-952 cell lines were similar with the Ishikawa cells. The numbers of cells penetrating the filter membrane into the lower chamber in Slug-shRNA and FoxC2-shRNA groups were 148.72±6.54 and 136.48±5.22, respectively. The numbers of cells penetrating Matrigel into the lower chamber in Slug-shRNA and FoxC2-shRNA groups were 76.42±5.63 and 77.82±7.32, respectively. While those in the control group 198.52±9.72 in migration assay and 178.63±6.38 in invasion assay (Figure 6). Compared with the control group, the numbers of cells entering the lower chamber in Slug-shRNA and FoxC2-shRNA groups in both cell lines were significantly reduced (P<0.05).

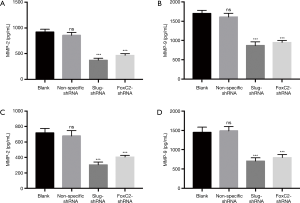
Slug-shRNA and FoxC2-shRNA reduced MMP-2 and MMP-9 in endometrial carcinoma cells
Subsequently, we compared the MMP-2 and MMP-9 of the cell culture supernatant in each group of both cell lines by ELISA assay. As shown in Figure 7, the expressions of MMP-2 and MMP-9 after shRNA-Slug and shRNA-FoxC2 transfection were significantly reduced. This is consistent with the results of our previous migration and invasion results.
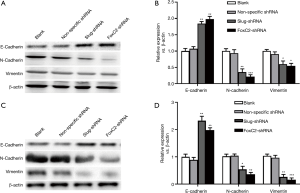
shRNA-Slug and shRNA-FoxC2 inhibited EMT in endometrial carcinoma
To gain insights into the mechanism underlying the Slug and FoxC2 inhibiting endometrial carcinoma migration and invasion, we evaluated the protein level of EMT related protein including E-cadherin, N-cadherin and Vimentin by Western blotting. As shown in Figure 8, after transfection of shRNA-Slug and shRNA-FoxC2 in Ishiyama and RL-952 endometrial carcinoma cells, the expression of E-cadherin increased while the expression of N-cadherin and Vimentin decreased. These results indicated that EMT capability is suppressed by shRNA-Slug and shRNA-FoxC2.
Discussion
In the current study, we found that Slug and FoxC2 expressions in endometrial carcinoma tissues were remarkably increased. In addition, Slug and FoxC2 silences by shRNA significantly inhibited endometrial carcinoma proliferation and metastasis, which may be related to the downregulation of MMPs protein expression and inhibition of EMT.
As a member of the Zinc finger transcription factor family members, Slug is mainly involved in neoplasm malignant phenotype regulation, and could also promote EMT, which is closely related to tumor cell migration and invasion (2,5). Recent researches have indicated that, Slug antisense could prevent EMT, indicating that, the Slug gene could act a treatment target for tumor invasion and metastasis (6-8).
FoxC2 is also referred to as mesenchyme forkhead 1 (MFH1), which belongs to the forkhead transcription factor family member and is encoded by genes located at human chromosome 16q24.1 (9,10). It has been highlighted that FoxC2 plays a central role in multiple signaling pathways, which are closely related to EMT and could further promote angiogenesis in tumor development (11,12).
In our current study, we found that the positive expression rates of Slug and FoxC2 proteins in endometrial carcinoma of 68.75% and 59.34%, respectively, which were remarkably elevated compared with that in normal endometrial tissue group (25.00%). Besides, the Slug and FoxC2 expressions were evidently correlated with the endometrial carcinoma development and poor prognosis (P<0.05). This suggests that Slug and FoxC2 may play a crucial role in endometrial carcinoma metastasis and Slug and FoxC2 could be used as an indicator for the prognosis of endometrial carcinoma.
In order to verify the Slug and FoxC2 roles in endometrial carcinoma, Ishikawa and RL-962 endometrial carcinoma cells were transfected with shRNA-Slug and shRNA-FoxC2 to silence the expression of Slug and FoxC2 respectively, which were confirmed by the fluorescence microscopy, Western blotting, and RT-PCR. Cell proliferation, invasion and migration were significantly inhibited by shRNA-Slug and shRNA-FoxC2 in both cell lines. The matrix metalloproteinases (MMP) family is a class of Zn2+ and Ca2+ -dependent endopeptidases that play an important role in multiple pathological processes such as inflammation, tumors, and cardiovascular diseases. It has found that MMP-2 and MMP-9 can promote tumor neovascularization in vivo, and upregulation of MMP-2 and MMP-9 in stromal cells could significantly increase the tumor cell metastasis (13). In the current study, our results also highlighted that the expressions of MMP-2 and MMP-9 in both cell lines after shRNA-Slug and shRNA-FoxC2 transfection was significantly decreased compared with the control group.
Infiltration and metastasis are also the main causes of treatment failure and poor clinical prognosis of endometrial carcinoma. It has been reported that metastasizes of endometrial carcinoma related to by EMT was induced by Slug and FoxC2 (14-19). EMT was an originally physiological process that occurred during the development of mammalian embryos, and recent research showed that EMT played an important role in the metastasis of tumors. Epithelial-derived tumor cells can be transformed into tumor cells with stronger migration ability and the enhanced invasions could also be promoted by interstitial cells. EMT could be activated by both intracellular signaling pathways and integrin signaling pathways, such as Slug, Snail, Twist and FoxC2 (20-25). Then the expressions EMT markers (such as Slug, Snail, and FoxC2) in tumor cells will be significantly increased, which will enhance cell invasiveness and lead to transformation into tumor cells (11,26,27), and the migration and resistance to apoptosis of the tumor epithelial cells will be enhanced (28-30). As EMT markers, E-cadherin, N-cadherin and Vimentin were analyzed in each group in both cell lines. We found that, after transfection of shRNA-Slug and shRNA-FoxC2, the expression of E-cadherin was markedly increased while the N-cadherin and Vimentin expression was significantly decreased, which was in consistence with previous studies (5,31), indicating the suppression of EMT by shRNA-Slug and shRNA-FoxC2.
Conclusions
In summary, our current study found that, compared with normal endometrial tissues, the Slug and FoxC2 expression levels in endometrial carcinoma tissues were remarkably increased. Interfering with Slug and FoxC2 through shRNAs can effectively suppress the in vitro proliferation, invasion, and migration of Ishikawa and RL-952cells, which may be related to the downregulation of MMPs protein and inhibition of EMT. Further studies will more focus on the detailed mechanism of the regulation of EMT in endometrial carcinoma by Slug and FoxC2.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/tcr-20-809
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-809
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-809). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of The First Affiliated Hospital of Bengbu Medical College (No. BBMEC-2018-10) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Phillips S, Kuperwasser C. SLUG: Critical regulator of epithelial cell identity in breast development and cancer. Cell Adh Migr 2014;8:578-87. [Crossref] [PubMed]
- Kihara A, Wakana K, Kubota T, et al. SLUG expression is an indicator of tumour recurrence in high-grade endometrial carcinomas. Histopathology 2016;69:374-82. [Crossref] [PubMed]
- Zhao Z, Sun YS, Chen W, et al. Hispolon inhibits breast cancer cell migration by reversal of epithelial-to-mesenchymal transition via suppressing the ROS/ERK/Slug/E-cadherin pathway. Oncol Rep 2016;35:896-904. [Crossref] [PubMed]
- Liu B, Han SM, Tang XY, et al. Overexpressed FoxC2 in ovarian cancer enhances the epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Oncol Rep 2014;31:2545-54. [Crossref] [PubMed]
- Du J, Zhang XP, Zhou HJ, et al. Alex3 suppresses non-small cell lung cancer invasion via AKT/Slug/E-cadherin pathway. Tumour Biol 2017;39:1010428317701441. [Crossref] [PubMed]
- Wu DW, Lee MC, Hsu NY, et al. FHIT loss confers cisplatin resistance in lung cancer via the AKT/NF-κB/Slug-mediated PUMA reduction. Oncogene 2015;34:2505. [Crossref] [PubMed]
- Anzai E, Hirata K, Shibazaki M, et al. FOXA1 Induces E-Cadherin Expression at the Protein Level via Suppression of Slug in Epithelial Breast Cancer Cells. Biol Pharm Bull 2017;40:1483-89. [Crossref] [PubMed]
- Uygur B, Abramo K, Leikina E, et al. SLUG is a Direct Transcriptional Repressor of PTEN Tumor Suppressor. Prostate 2015;75:907-16. [Crossref] [PubMed]
- Sánchez-Duffhues G, de Vinuesa AG, Lindeman JH, et al. SLUG Is Expressed in Endothelial Cells Lacking Primary Cilia to Promote Cellular Calcification. Arterioscler Thromb Vasc Biol 2015;35:616-27. [Crossref] [PubMed]
- Jiang F, Zhou L, Wei C, et al. Slug inhibition increases radiosensitivity of oral squamous cell carcinoma cells by upregulating PUMA. Int J Oncol 2016;49:709-19. [Crossref] [PubMed]
- Zhang CL, Zhu KP, Ma XL. Antisense lncRNA FoxC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FoxC2. Cancer Lett 2017;396:66-75. [Crossref] [PubMed]
- Paranjape AN, Soundararajan R, Werden SJ, et al. Inhibition of FoxC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties. Oncogene 2016;35:5963-76. [Crossref] [PubMed]
- Cui YM, Jiao HL, Ye YP, et al. FoxC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene 2015;34:4379-90. [Crossref] [PubMed]
- Jiang W, Fan H, Qian C, et al. Prognostic value of high FoxC2 expression in resectable non-small cell lung cancer, alone or in combination with E-cadherin expression. BMC Cancer 2016;16:16. [Crossref] [PubMed]
- Wu B, Wei J, Hu Z, et al. Slug silencing inhibited perineural invasion through regulation of EMMPRIN expression in human salivary adenoid cystic carcinoma. Tumour Biol 2016;37:2161-69. [Crossref] [PubMed]
- Zheng M, Jiang YP, Chen W, et al. Snail and Slug collaborate on EMT and tumor metastasis through miR-101-mediated EZH2 axis in oral tongue squamous cell carcinoma. Oncotarget 2015;6:6797-810. [Crossref] [PubMed]
- Cappellesso R, Marioni G, Crescenzi M, et al. The prognostic role of the epithelial-mesenchymal transition markers E-cadherin and Slug in laryngeal squamous cell carcinoma. Histopathology 2015;67:491-500. [Crossref] [PubMed]
- Tsukasa K, Ding Q, Yoshimitsu M, et al. Slug contributes to gemcitabine resistance through epithelial-mesenchymal transition in CD133(+) pancreatic cancer cells. Hum Cell 2015;28:167-74. [Crossref] [PubMed]
- Cai J, Tian AX, Wang QS, et al. FOXF2 suppresses the FoxC2-mediated epithelial mesenchymal transition and multidrug resistance of basal-like breast cancer. Cancer Lett 2015;367:129-37. [Crossref] [PubMed]
- Zhou Z, Zhang L, Xie B, et al. FoxC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett 2015;363:137-45. [Crossref] [PubMed]
- Liu B, Han SM, Tang XY, et al. Overexpressed FoxC2 in ovarian cancer enhances the epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Oncol Rep 2014;31:2545-54. [Crossref] [PubMed]
- Merikallio H, Turpeenniemi-Hujanen TT, Paakko P, et al. Slug is associated with poor survival in squamous cell carcinoma of the lung. Int J Clin Exp Pathol 2014;7:5846-54. [PubMed]
- Zhang T, Liang L, Liu X, et al. TGF beta 1-Smad3-Jagged1-Notch1-Slug signaling pathway takes part in tumorigenesis and progress of tongue squamous cell carcinoma. J Oral Pathol Med 2016;45:486-93. [Crossref] [PubMed]
- Sun TY, Xie HJ, Li Z, et al. Expression of FoxC2 in renal cell carcinoma and its relationship to clinical pathological features. Int J Clin Exp Med 2015;8:13388-92. [PubMed]
- Li C, Ding H, Tian J, et al. Forkhead Box Protein C2 (FoxC2) Promotes the Resistance of Human Ovarian Cancer Cells to Cisplatin In Vitro and In Vivo. Cell Physiol Biochem 2016;39:242-52. [Crossref] [PubMed]
- Xue M, Zhu FY, Chen L, et al. HoxB9 promotes the migration and invasion via TGF-beta1/Smad2/Slug signaling pathway in oral squamous cell carcinoma. Am J Transl Res 2017;9:1151-61. [PubMed]
- Yang J, Ren YX, Shang YT, et al. Effect of Slug and Sox9 on the Invasion and Metastasis of Nasopharyngeal Carcinoma Stem Cells. J Kunming Med Univ 2016;37:52-55.
- Yang C, Cui XX, Dai XQ, et al. Downregulation of FoxC2 enhances apoptosis induced by 5-fluorouracil through activation of MAPK and AKT pathways in colorectal cancer. Oncol Lett 2016;11:1549-54. [Crossref] [PubMed]
- Xu T, Fan B, Lv CJ, et al. Slug mediates nasopharyngeal carcinoma radio résistance via downregulation of PUMA in a p53-dependent and -independent manner. Oncol Rep 2015;33:2631-38. [Crossref] [PubMed]
- Jittreetat T, Shin YS, Hwang HS, et al. Tolfenamic Acid Inhibits the Proliferation, Migration, and Invasion of Nasopharyngeal Carcinoma: Involvement of p38-Mediated Down-Regulation of Slug. Yonsei Med J 2016;57:588-98. [Crossref] [PubMed]
- Kanady JD, Munger SJ, Witte MH, et al. Combining FoxC2 and Connexin37 deletions in mice leads to severe defects in lymphatic vascular growth and remodeling. Dev Biol 2015;405:33-46. [Crossref] [PubMed]


