Long-term survival of patients with anaplastic thyroid cancer after multimodal treatment
Introduction
Anaplastic thyroid cancer (ATC) is extremely aggressive, with rapid disease progression, very short survival, and extremely poor treatment outcomes (1). In addition, although ATC is rare, accounting for only 1–2% of all thyroid cancers, deaths owing to ATC account for up to 50% of all deaths caused by thyroid cancer (2,3). Moreover, the 1- and 10-year survival rates are 10–20% and less than 5%, respectively, although the reliability of the diagnoses in these long-term survivors is debatable (4,5). Therefore, long-term survival is rare, having been reported in only a small number of selected patients.
The mechanisms of ATC progression are not completely understood. Surgery is the most effective treatment to improve prognosis; however, most cases cannot be managed because of the extent of local disease or distant metastasis at the time of initial diagnosis (6,7). Current chemotherapy regimens also have limited efficacy and have not resulted in a significant increase in overall survival (OS) (8).
ATC is currently treated with chemotherapy, radiotherapy, targeted therapy, and/or surgery. Early mutations in BRAF and RAS have been reported in 25% and 28% of cases, respectively. BRAF V600E-positive malignancies were treated with the BRAF inhibitor dabrafenib (150 mg twice daily) plus the MEK inhibitor trametinib (2 mg once daily) (9). Other rare mutations and genetic aberrations, such as ALK translocations, may also be targeted via specific drugs (10). Because only less than 30% of patients with ATC have genetic mutations that can be treated via targeted therapy, extended molecular profiling of ATC is strongly encouraged as it may reveal promising possibilities for targeted therapy. Accordingly, the use of targeted therapy, immunotherapy, chemotherapy and/or radiotherapy, in combination or in sequence, for multidisciplinary ATC management may improve patient outcomes (11).
The median OS of patients with ATC is 0.5 to 6 months worldwide, despite multimodal treatment (12,13); moreover, even after aggressive treatments, the median OS has not changed significantly in several decades (14). Accordingly, the current study aimed to describe our experience with the management of 23 patients with ATC who showed long-term survival of more than 1 year and to determine the effective treatment that had an impact on the survival of more than 1 year.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1364).
Methods
Patients
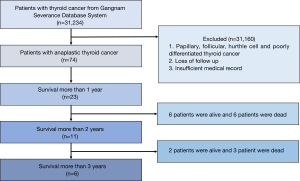
The medical records of 23 patients with pathologically proven ATC who survived for more than 1 year were retrospectively identified and reviewed from the single-centre database system of the Gangnam Severance Hospital between January 2003 and December 2018. The management and outcome data of the 23 patients with a definitive histological diagnosis of ATC were reviewed (Figure 1). The American Joint Committee on Cancer (AJCC) staging system (8th edition) was used for tumour staging (15). The final pathologic confirmation was performed via surgery in 19 patients and via fine-needle aspiration biopsy in 4 patients. The histologic findings were examined by a pathologist to exclude the possibility of poorly differentiated carcinoma. The patient records were anonymised and de-identified prior to analysis, and hence, informed consent was not obtained from each participant. The study was approved by Institutional Review Board of Yonsei University (No. 3-2018-0135) and conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Surgery for ATC included radical resection for patients with resectable tumours and partial/palliative resection for patients with unresectable tumours. Radical resection usually included total thyroidectomy because ATC commonly shows aggressive behaviour and extensive tumour burden at presentation (1). Partial thyroidectomy was performed when the patient had a low tumour burden and when the tumour was focally limited to one lobe of the thyroid. For patients with clinically positive cervical lymph nodes, level II to VI neck node dissection was also performed. When patients could not undergo surgery owing to poor general condition, old age, extensive tumour burden, or refusal to undergo surgery, tracheostomy and tumour biopsy were performed.
Among chemotherapeutic regimens, paclitaxel-based concurrent chemoradiotherapy (CCRT) was usually administered before and after surgery in our institution according to the clinicians’ preference and patients’ condition. If patients could not tolerate CCRT, physicians occasionally decided to administer RT only. Sequential chemotherapy with paclitaxel after CCRT was also administered if necessary.
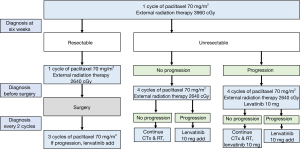
Thyrosine kinase inhibitor, LENVIMA®[lenvatinib] was administered according to ATC Yonsei university medical center (YUMC) protocol. We administered lenvatinib 10 mg a day, to patients who had progression after underwent surgery and 3 cycles of adjuvant paclitaxel mono chemotherapy.
If unresectable and no progression, the patient underwent 4 cycles of adjuvant paclitaxel mono chemotherapy and external radiation therapy. And then the cancer was progression, we add lenvatinib 10 mg a day. If unresectable and progression, the patient underwent 4 cycles of adjuvant paclitaxel mono chemotherapy and external radiation therapy and lenvatinib is orally administered 10 mg a day. If more progression, we add lenvatinib 10 mg a day.
Statistical analysis
OS was defined as the time from the date of the first treatment to the time of death owing to any cause or last follow-up. Survival rates were calculated using the Kaplan-Meier method. All these analyses were performed with the statistical program package SPSS for windows, version 22.
Results
Clinical and pathological characteristics
The demographic data of the 23 included patients are presented in Tables 1,2. Among the 23 patients, 11 were men and 12 were women, with a median age of 58.3 years (range, 27–78 years). A total of 19 patients (82.6%) had symptoms. Thirteen patients (68.4%) had a palpable mass, 4 (21.1%) had localised pain, and 2 (10.5%) had dyspnoea. Overall, 9 patients (39.1%) had distant metastasis (Table 2).
Table 1
| Patient | Sex | Age (y) | Stage | Surgery | CTx administered | RTx dose | TKI administered | Survival status | Survival days |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 75 | 4c | R0 | No | NA | No | Dead | 646 |
| 2 | Male | 47 | 4b | R0 | No | NA | No | Alive | 3,525 |
| 3 | Female | 67 | 4c | R0 | No | 5,580 | No | Dead | 1,090 |
| 4 | Female | 37 | 4b | R0 | No | NA | No | Alive | 3,058 |
| 5 | Female | 61 | 4b | R0 | No | 6,600 | No | Alive | 1,553 |
| 6 | Male | 66 | 4b | No | Yes | 7,800 | Yes | Dead | 506 |
| 7 | Female | 79 | 4b | R1 | No | NA | No | Dead | 483 |
| 8 | Female | 69 | 4c | R0 | Yes | 7,412 | Yes | Dead | 633 |
| 9 | Female | 72 | 4b | No | Yes | 3,360 | Yes | Dead | 1,316 |
| 10 | Male | 59 | 4c | R0 | No | 3,500 | Yes | Dead | 913 |
| 11 | Female | 55 | 4b | R0 | Yes | 4,400 | No | Alive | 1,340 |
| 12 | Female | 51 | 4b | R0 | No | NA | No | Alive | 1,205 |
| 13 | Male | 74 | 4b | R1 | Yes | 6,510 | No | Alive | 831 |
| 14 | Male | 67 | 4c | No | Yes | 6,000 | No | Dead | 546 |
| 15 | Female | 64 | 4c | R1 | Yes | 7,880 | No | Alive | 630 |
| 16 | Male | 59 | 4c | No | Yes | 5,400 | No | Dead | 410 |
| 17 | Male | 60 | 4c | R0 | Yes | 6,000 | Yes | Dead | 839 |
| 18 | Male | 56 | 4b | R0 | Yes | 5,880 | No | Alive | 742 |
| 19 | Male | 28 | 4b | R0 | Yes | 6,000 | No | Alive | 631 |
| 20 | Female | 64 | 4c | R2 | Yes | 3,080 | Yes | Alive | 390 |
| 21 | Male | 42 | 4b | R0 | Yes | 6,000 | No | Alive | 422 |
| 22 | Female | 53 | 4b | R0 | Yes | 4,000 | No | Alive | 419 |
| 23 | Male | 41 | 4b | R2 | Yes | 4,000 | No | Alive | 376 |
CTx, chemotherapy; RTx, radiotherapy; TKI, tyrosine kinase inhibitor; NA, not administered.
Table 2
| Variables | n (%) |
|---|---|
| Number | 23 |
| Age (years), mean ± SD, (range) | 58.5±13.0 [27–78] |
| Male:female ratio | 11:12 (47.8:52.2) |
| Clinical presentation | 19 (82.6) |
| Palpable mass | 13 (68.4) |
| Hoarseness | 1 (5.3) |
| Localized pain | 4 (21.1) |
| Dyspnoea | 2 (10.5) |
| Dysphagia | 1 (5.3) |
| Motor nerve palsy | 1 (5.3) |
| Every weight loss | 1 (5.3) |
| Eastern Cooperative Oncology Group performance status score | |
| 0:1:2 ratio | 14 (78.3):4 (17.4):1 (4.3) |
| Distant metastasis | 9 (39.1) |
| Lungs | 9 (100.0) |
| Bone | 2 (22.2) |
| Brain | 2 (22.2) |
| Adrenal gland | 1 (11.1) |
| Stage at diagnosis | |
| IVB:IVC | 14 (60.9):9 (39.1) |
Data are given as n (%) unless indicated otherwise.
The Eastern Cooperative Oncology Group (ECOG) performance status score was 0 in 14 patients (78.3%), 1 in 4 patients (17.4%), and 2 in 1 patient (4.3%). At the time of diagnosis, 14 patients (60.9%) had stage IVB disease and 9 patients (39.1%) had stage IVC disease according to the AJCC 8th edition (Table 2).
Treatment characteristics are shown in Table 3. In total, 14 patients were treated with a combination of surgery and radiotherapy with or without chemotherapy. Only 5 patients were treated with surgery alone. Overall, 15 patients underwent R0 resection, 2 underwent R1 resection, and 2 underwent R2 resection.
Table 3
| Variables | n (%) |
|---|---|
| Treatment modality | |
| Surgery + RTx + CTx + TKI | 3 (13.0) |
| Surgery + RTx + CTx | 8 (34.8) |
| Surgery + RTx + TKI | 1 (4.3) |
| Surgery + RTx | 2 (8.7) |
| Surgery only | 5 (21.7) |
| RT + CTx + TKI | 2 (8.7) |
| RT + CTx | 2 (8.7) |
| Surgery | 19 (82.6) |
| R0 resection | 15 (78.9) |
| R1 resection | 2 (10.5) |
| R2 resection | 2 (10.5) |
Data are given as n (%) unless indicated otherwise.
Survival rates
The median survival of patients was 1,090 days. The median follow-up was 646 days. The 2- and 3-year survival rates were 59.7% and 35.8%, respectively (Figure 2). Overall, 10 patients died: 7 owing to local disease and 3 owing to distant metastasis (Table 4).
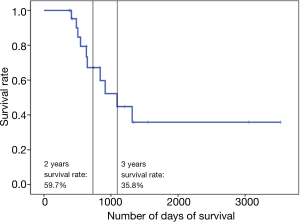
Table 4
| Variables | |
|---|---|
| Median follow-up, days | 646 [376–3,525] |
| Median survival, days | 1,090 [376–3,525] |
| 2-year survival rate (%) | 59.7 |
| 3-year survival rate (%) | 35.8 |
| Number of patients who died | 10 (43.5) |
| Cause of death | |
| Local disease | 3 (30.0) |
| Distant metastasis | 7 (70.0) |
Discussion
ATC is more common in women than in men. On evaluating the data of patients in the Surveillance, Epidemiology, and End Results database from 1973 to 2014, a total of 1,527 patients were diagnosed with ATC in the SEER 18 registries (16). Of 1,527 patients, 959 (62.8%) were women and 568 (37.2%) were men; the mean age was 70.5 years (16). However, in the current study, there were no differences considering the patient sex, and only 4 patients were older than 70 years; the median age was only 58.3 years (range, 27–78 years).
The ECOG performance status score was 0 in 14 patients (78.3%), 1 in 4 patients (17.4%), and 2 in 1 patient (4.3%). In fact, a lower ECOG performance status score is associated with better treatment tolerance and smooth recovery.
The locoregional invasiveness of ATC may cause compressive symptoms such as dysphagia, dyspnoea, stridor, and pain. In the current study, 19 patients (82.6%) had symptoms on diagnosis. This was similar to the result of a retrospective study in which the majority of patients (86.1%) presented with compressive symptoms (17). Therefore, the presence of compressive symptoms does not affect OS nor does it result in early diagnosis despite aggressive disease progression. In fact, in the current study, all the patients who did not undergo R0 resection or surgery were treated with radiotherapy, which reduced local recurrence, thereby resulting in longer OS.
More than 60% of patients with ATC present with distant metastasis (18). In the current study, only 9 patients (39.1%) had distant metastasis. All the 9 patients had distant metastasis to the lungs, and they also had bone, brain, and adrenal metastasis. When patients with ATC do not have distant metastasis, the possibility of performing surgery is higher; therefore, there is no evidence that indicates that distant metastasis increased the OS and complete remission rate.
In the current study, 82.6% of the patients underwent surgery and received multimodal therapy. A total of 15 patients underwent R0 resection, 2 underwent R1 resection, and 2 underwent R2 resection. Early maximal debulking surgery or R0 resection and systemic chemotherapy and radiotherapy may influence the survival of patients (12,18,19). However, the benefit of aggressive surgery is often of limited clinical significance in advanced cases and is possibly outweighed by the associated morbidity. Moreover, negative margins may not have any survival benefit (20). In fact, surgery alone may not have any survival benefit. However, R0 resection combined with multimodal therapy increased OS. The 2- and 3-year survival rates were 59.7% and 35.8%, respectively, indicating the possibility of complete remission in patients with ATC. In fact, the possibility of complete remission is higher in patients who survive for more than 1 year than in other patients. In our treatment centre, we have administered the ATC YUMC protocol (Figure 3) for patients with ATC since 2015, which is why it is difficult to compare the results obtained for our patients to those obtained for other patients because of the use of a unified treatment protocol. Therefore, in the future, a comparison study is required for evaluating patients with ATC before and after the use of the ATC YUMC protocol.
This study had several limitations. This was an observational study, and the results were dependent on the completeness and accuracy of the medical records used. In addition, as this study only included patients from a single centre, the generalizability of the current findings may be limited. Moreover, in case of cancers with a high mortality such as pancreatic cancer, it is preferable to review the long-term survival of patients. Nevertheless, as the current study aimed to identify the characteristics of patients with ATC who survived for more than 1 year, the results could be the basis for further research. Furthermore, the findings of this study will help in increasing the rate of treatment for patients with ATC.
Conclusions
In conclusion, Although ATC is typically an incurable disease, patients with ATC who underwent multimodality treatments including resection, chemotherapy, radiotherapy, and thyrosine kinase inhibitors may be survive more than 1 year.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1364
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1364
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1364). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The patient records were anonymised and de-identified prior to analysis, and hence, informed consent was not obtained from each participant. The study was approved by Institutional Review Board of Yonsei University (No. 3-2018-0135) and conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Passler C, Scheuba C, Prager G, et al. Anaplastic (undifferentiated) thyroid carcinoma (ATC). A retrospective analysis. Langenbecks Arch Surg 1999;384:284-93. [Crossref] [PubMed]
- Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol 2006;13:453-64. [Crossref] [PubMed]
- Chintakuntlawar AV, Foote RL, Kasperbauer JL, et al. Diagnosis and Management of Anaplastic Thyroid Cancer. Endocrinol Metab Clin North Am 2019;48:269-84. [Crossref] [PubMed]
- Kebebew E, Greenspan FS, Clark OH, et al. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 2005;103:1330-5. [Crossref] [PubMed]
- McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 2001;130:1028-34. [Crossref] [PubMed]
- Koyama S, Miyake N, Fujiwara K, et al. Lenvatinib for Anaplastic Thyroid Cancer and Lenvatinib-Induced Thyroid Dysfunction. Eur Thyroid J 2018;7:139-44. [Crossref] [PubMed]
- Kim SM, Park KC, Jeon JY, et al. Potential anti-cancer effect of N-hydroxy-7-(2-naphthylthio) heptanomide (HNHA), a novel histone deacetylase inhibitor, for the treatment of thyroid cancer. BMC Cancer 2015;15:1003. [Crossref] [PubMed]
- Kojic SL, Strugnell SS, Wiseman SM. Anaplastic thyroid cancer: a comprehensive review of novel therapy. Expert Rev Anticancer Ther 2011;11:387-402. [Crossref] [PubMed]
- Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 2018;36:7-13. [Crossref] [PubMed]
- Pozdeyev N, Gay LM, Sokol ES, et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin Cancer Res 2018;24:3059-68. [Crossref] [PubMed]
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1856-83. [Crossref] [PubMed]
- Yau T, Lo CY, Epstein RJ, et al. Treatment outcomes in anaplastic thyroid carcinoma: survival improvement in young patients with localized disease treated by combination of surgery and radiotherapy. Ann Surg Oncol 2008;15:2500-5. [Crossref] [PubMed]
- Mohebati A, Dilorenzo M, Palmer F, et al. Anaplastic thyroid carcinoma: a 25-year single-institution experience. Ann Surg Oncol 2014;21:1665-70. [Crossref] [PubMed]
- Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg 2015;4:44-51. [PubMed]
- Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017;27:751-6.
- Janz TA, Neskey DM, Nguyen SA, et al. Is the incidence of anaplastic thyroid cancer increasing: A population based epidemiology study. World J Otorhinolaryngol Head Neck Surg 2019;5:34-40. [Crossref] [PubMed]
- Simões-Pereira J, Capitão R, Limbert E, et al. Anaplastic Thyroid Cancer: Clinical Picture of the Last Two Decades at a Single Oncology Referral Centre and Novel Therapeutic Options. Cancers (Basel) 2019;11:1188. [Crossref] [PubMed]
- Brignardello E, Palestini N, Felicetti F, et al. Early surgery and survival of patients with anaplastic thyroid carcinoma: analysis of a case series referred to a single institution between 1999 and 2012. Thyroid 2014;24:1600-6. [Crossref] [PubMed]
- He X, Li D, Hu C, et al. Outcome after intensity modulated radiotherapy for anaplastic thyroid carcinoma. BMC Cancer 2014;14:235. [Crossref] [PubMed]
- Hu S, Helman SN, Hanly E, et al. The role of surgery in anaplastic thyroid cancer: A systematic review. Am J Otolaryngol 2017;38:337-50. [Crossref] [PubMed]