
Detection and prognostic value of intratumoral and peritumoral lymphangiogenesis in colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most common malignancies and is considered the third leading cause of cancer-related deaths worldwide. CRC with lymphatic invasion is one of the critical prognostic factors in lymph node metastasis (1). Given this, clarifying the molecular and cellular mechanisms underlying the process of metastasis, a major avenue of cancer research, is needed to help identify a new therapeutic target in the treatment of CRC.
It has been reported that there is a close relationship between increased lymphangiogenesis and metastatic spread in studies of other human cancers (2-6). For example, in head and neck cancers, malignant melanoma, and prostate and breast cancer, increased intratumoral or peritumoral lymphatic vessel density (LVD) has been found to be associated with metastatic spread and poorer prognosis. It has also been suggested that high LVD in CRC is associated with lymphatic metastasis and poor prognosis (5,7,8). Nevertheless, the clinical significance of intratumoral or peritumoral lymphatics in CRC remains unclear in these studies, as is the case for studies of other tumors (9).
There is still debate concerning the effect of intratumoral or peritumoral lymphatics on the progression and prognosis of different tumors (10). In pancreatic ductal adenocarcinoma, in which early lymph node metastasis is common, neither intratumoral nor peritumoral lymphangiogenesis was present or detected (11). In gastric cancer, thyroid papillary carcinoma, and squamous cell carcinoma of the head, neck, and esophagus, intratumoral LVD (LVDit) was predictive of lymphatic metastasis (12-15). Meanwhile, peritumoral LVD (LVDpt) has been associated with lymph node metastases in cutaneous melanoma, breast cancer, prostate adenocarcinoma, and uterine cervix carcinoma (3-6). However, the function of different lymphatic vessels in CRC is still elusive and controversial. Some studies have suggested that LVDit is related to tumor progression and prognosis (16,17), whereas other studies have presented conflicting results (18-20).
Thus, the aim of the present study was to detect intratumoral and peritumoral lymphangiogenesis and explore the relationship between clinicopathological parameters, including LVDit or LVDpt, lymph node metastasis, pathological stage, and prognostic factors in CRC.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1038).
Methods
CRC samples
The present study involved 120 primary CRC patients who underwent surgical resection at the Department of Gastroenterological Surgery, Peking University People’s Hospital, Beijing, China, between September 2010 and April 2012. None of the patients had undergone preoperative radiotherapy and chemotherapy. Patients were followed up clinically for more than 5 years postoperatively. The time range of follow-up was 1–78 months, with an average of 53 months. All the samples were fixed using 10% formalin for 24 hours, and were then embedded with paraffin wax. After being cut into 4-µm sections, the samples were treated with hematoxylin and eosin (HE) staining and observed under a microscope by two experienced pathologists according to the criteria of the Union for International Cancer Control. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research was approved by the Ethics Committee of Peking University People’s Hospital (No. 2020PBH006-01), and informed consent was taken from all the patients.
Immunohistochemical staining
The patients’ biopsy sections were immunohistochemically stained using a streptavidin-peroxidase technique (Beijing Zhongshan Golden Bridge Biological Technology). Briefly, the embedded sections were deparaffinized in a graded series of ethanol and 100% xylene; 0.3% hydrogen peroxide in methanol was used for 10 minutes at room temperature to block endogenous peroxidase activity. After treatment with 10% normal rabbit serum for 10 minutes, sections were stained overnight with a mouse antihuman podoplanin monoclonal antibody (AngioBio) as the primary antibody at 4 °C. Each slide was incubated with antimouse immunoglobulin G antibody for 10 minutes and streptavidin-biotinylated horseradish peroxidase complex for 5 minutes. Diaminobenzidine and Mayer’s hematoxylin solution were used as a chromogen and nuclear counterstain, respectively. Phosphate-buffered saline (PBS) without a primary antibody was added for a negative control.
LVD assessment
LVD quantification was tested as previously described (21). A microvessel was considered to be a single endothelial cell or a cluster of endothelial cells positive for podoplanin, and was located around a visible lumen, which was easily separated from adjacent microvessels and from other connective tissue components. Intratumoral lymphatic vessels were defined as those within the tumor cell islets, and peritumoral lymphatic vessels as those in the periphery within 2 mm of tumors adjacent to the invasion front. Briefly, the three most vascularized areas examined by podoplanin were primarily visualized (so-called hotspots) under a 40× field. Vessels in each of these areas were then detected under a 200× field. The mean values of three 200× field counts in this section were defined as the LVDpt or LVDit. The 120 cases were divided into two groups (LVDpt or LVDit group) in terms of the mean level of LVD.
Statistical analyses
All statistical analyses were carried out with SPSS version 22.0 software. Statistical comparisons were performed using unpaired two-tailed Student’s t-test or one-way analysis of variance (ANOVA) as appropriate. Overall survival curves were obtained by the Kaplan-Meier method, and the statistical significance of differences was evaluated by log-rank test. Univariate and multivariate analyses were carried out using the Cox proportional hazards model. Differences at P<0.05 were considered statistically significant.
Results
Intratumoral and peritumoral lymphatic vessels in CRC
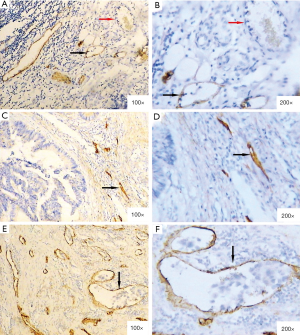
The staining was specifically positive in lymphatic endothelial cells and negative in vascular endothelial cells, using podoplanin monoclonal antibody (Figure 1A,B). Intratumoral lymphatic vessels were usually small, collapsed, and irregular (Figure 1C,D). In contrast, the peritumoral lymphatic vessels were generally large and dilated, and were occasionally involved in tumor cell clusters (Figure 1E,F). Overall, the mean LVDpt was higher than the mean LVDit (18.99±6.89 vs. 9.91±4.25; P<0.001).
Relationship of LVDit and LVDpt with clinicopathological parameters in CRC
The correlations of LVDit and LVDpt with clinicopathological findings are summarized in Table 1. High LVDit was found to be significantly correlated with larger tumor size (P=0.009) and poor differentiation (P=0.023). In contrast, high LVDpt had a significant correlation with lymph node metastasis (P<0.001) and late tumor-node-metastasis (TNM) stage (P=0.004). No significant correlations were found between LVDit or LVDpt and other characteristics, including sex, age, tumor location, T-stage, and distant metastasis.
Table 1
| Characteristics | N | LVDit (mean ± SD) | P value | LVDpt (mean ± SD) | P value |
|---|---|---|---|---|---|
| Age at presentation (years) | 0.314 | 0.875 | |||
| <65 | 62 | 10.29±4.17 | 18.89±6.72 | ||
| ≥65 | 58 | 9.51±4.32 | 19.09±7.12 | ||
| Sex | 0.062 | 0.668 | |||
| Male | 74 | 10.48±4.34 | 19.20±7.20 | ||
| Female | 46 | 8.99±3.96 | 18.64±6.42 | ||
| Site of tumor | 0.156 | 0.488 | |||
| Colon | 75 | 10.33±4.25 | 19.33±7.03 | ||
| Rectum | 45 | 9.20±4.18 | 18.42±6.99 | ||
| Size of tumor | 0.009* | 0.516 | |||
| >5 cm | 35 | 11.49±4.23 | 19.63±7.54 | ||
| ≤5 cm | 85 | 9.26±4.11 | 18.73±6.63 | ||
| Histologic grade (differentiation) | 0.023* | 0.452 | |||
| Well, moderate | 21 | 11.81±3.73 | 16.04±7.41 | ||
| Poor, mucinous | 99 | 9.51±4.26 | 19.14±7.06 | ||
| Tumor status | 0.327 | 0.960 | |||
| T2 | 19 | 10.12±3.56 | 18.65±6.69 | ||
| T3 | 94 | 9.77±4.49 | 19.02±7.04 | ||
| T4 | 7 | 11.29±2.25 | 19.48±6.19 | ||
| Lymph node metastasis | 0.194 | <0.001* | |||
| Negative | 57 | 9.38±4.21 | 16.47±5.12 | ||
| Positive | 63 | 10.39±4.26 | 21.27±7.50 | ||
| Distant metastasis | 0.061 | 0.101 | |||
| Negative | 98 | 9.55±4.26 | 18.47±6.64 | ||
| Positive | 22 | 11.36±3.97 | 21.06±7.60 | ||
| TNM stage | 0.205 | 0.004* | |||
| I | 11 | 9.58±3.07 | 16.03±4.26 | ||
| II | 43 | 8.88±4.43 | 16.65±5.27 | ||
| III | 44 | 10.39±4.29 | 20.64±7.59 | ||
| IV | 22 | 11.12±4.06 | 21.73±7.59 |
*, P<0.05. LVD, lymphatic vessel density; CRC, colorectal cancer; LVDit, intratumoral LVD; LVDpt peritumoral LVD; SD, standard deviation.
Survival analysis
Kaplan-Meier analyses were performed for the overall survival of LVDit or LVDpt. The survival rate of patients characterized with low LVDit (n=56) was remarkably higher than that of patients with high LVDit (n=64, 5-year survival rate: 66.9% vs. 50%, P=0.036, log-rank) (Figure 2A). Furthermore, the survival rate was considerably high in patients with low LVDpt (n=79) compared with patients with high LVDpt (n=41, 5-year survival rate: 66.1% vs. 42%, P=0.016, log-rank) (Figure 2B).

In the univariate analysis, decreased survival was associated with poor histopathological differentiation, lymph node metastasis, distant metastasis, advanced clinical stage, and high LVDit or LVDpt (Table 2). In the multivariate analysis, poor histopathological differentiation (P=0.042), lymph node metastasis (P=0.017), and distant metastasis (P<0.001) were still regarded as the crucial independent prognostic factors of a decreased overall survival rate (Table 2).
Table 2
| Variables | Hazard ratio (95% CI) | P value |
|---|---|---|
| Univariate analysis | ||
| Histologic grade (differentiation) | 2.379 (1.168–4.847) | 0.017 |
| Well, moderate vs. poor, mucinous | ||
| Lymph node metastasis | 5.300 (2.527–11.113) | <0.001 |
| Negative vs. positive | ||
| Distant metastasis | 13.303 (6.906–25.625) | <0.001 |
| Negative vs. positive | ||
| TNM stage | 5.154 (2.381–11.161) | <0.001 |
| I, II vs. III, IV | ||
| LVDit | 1.897 (1.028–3.501) | 0.041 |
| Low vs. high | ||
| LVDpt | 2.061 (1.126–3.771) | 0.019 |
| Low vs. high | ||
| Multivariate analysis | ||
| Histological grade (differentiation) | 2.144 (1.029–4.467) | 0.042 |
| Well, moderate vs. poor, mucinous | ||
| Lymph node metastasis | 5.520 (1.364–22.341) | 0.017 |
| Negative vs. positive | ||
| Distant metastasis | 11.593 (5.224–25.730) | <0.001 |
| Negative vs. positive |
CI, confidence interval; TNM, tumor-node-metastasis; LVD, lymphatic vessel density; LVDit, intratumoral LVD; LVDpt, peritumoral LVD.
Discussion
The lymphatic vessel is the crucial metastasis pathway for the majority of cancers. Lymph node metastasis is a pivotal predictor of poor outcome, which implies the relevance of lymphatics to cancer biology (22). The discovery of novel markers for distinguishing blood and lymphatic vessels has facilitated the investigation of tumor-associated lymphangiogenesis and its potential function in tumor progression. Podoplanin is a special marker of lymphatic endothelial cells recommended for the evaluation of lymphangiogenesis in humans (21). In the present study, lymphatic vessels were immunostained with the podoplanin monoclonal antibody in CRC tissues, and we investigated the clinical significance of the podoplanin-positive lymphatic vessel counts. In the specimens used in our study, podoplanin expression was restricted to thin-walled lymphatic vessels with a single endothelial layer. Blood vessels with red blood cells failed to be stained, further demonstrating that podoplanin is a good lymphatic endothelial marker for the study of tumor-associated lymphangiogenesis.
It has been well established that lymphangiogenesis can occur in and around tumors (23). However, the functional significance of intratumoral and peritumoral lymphatics involved in the pathology of tumors remains controversial. In previous reports, lymphatic vessels were detected within the intratumoral area in gastric cancer (12), thyroid papillary carcinoma (13), and squamous cell carcinoma of the head, neck, and esophagus (14,15), and LVDit was found to be significantly associated with lymph node metastasis and a poorer prognosis compared to LVDpt. However, some other studies noted that high LVDpt was predictive of lymphatic involvement and poor prognosis in cutaneous melanoma, breast cancer, prostate adenocarcinoma, and uterine cervix carcinoma (3-6). Saad et al. reported that LVDit in CRC was related to both lymph node and liver metastases (16). Barresi et al. found that lymphangiogenesis was mostly located in the peritumoral area of CRC tissues, and LVDpt, rather than LVDit, was associated with lymph node metastasis in early CRC (18). Longatto-Filho et al. noted that LVDpt was correlated with hepatic metastasis, but not lymph node metastasis, in colon cancer, although LVDpt was lower than LVDit (20). These controversial findings might the result of a selective bias and a smaller number of samples. Most studies have mainly focused on a specified group of CRC patients with either early or late TNM stage. Based on our results from 120 CRC cases, spanning all TNM stages, we found that LVDpt, rather than LVDit, was substantially associated with lymph node metastasis, suggesting that LVDpt is more important in lymph node metastasis than LVDit.
The main location of lymphangiogenesis might vary in different types of tumors. In pancreatic ductal adenocarcinoma, lymphangiogenesis was not detected in either intratumoral or peritumoral areas (11). However, both LVDit and LVDpt increased in cutaneous melanoma (12). In another study, the majority of lymphatic vessels were located in intratumoral areas in squamous cell carcinoma of the head, neck, and esophagus (15); however, it was found to be the opposite for uterine cervix carcinoma (6). In our study, podoplanin-positive lymphatic vessels were observed both within the tumor mass and around the tumor periphery, and LVDpt was significantly higher than LVDit. Compared to intratumoral lymphatic vessels, peritumoral lymphatic vessels were generally large and dilated, thus further verifying the findings that in peritumoral areas of the tumor, both lymphangiogenesis and lymphatic vessel remodeling occurred, facilitating the entry of tumor cells into the lymphatics, and had functional importance in the spread of cancer (22). In addition, high LVDit was positively associated with larger tumor size and poor histopathological differentiation, indicating that intratumoral lymphangiogenesis may play a critical role in tumor growth and differentiation. These findings suggest that both intratumoral and peritumoral lymphangiogenesis contribute to CRC progression, but in a different manner.
In their study, Gao et al. found no relationship between LVD and other prognostic parameters, such as survival, in CRC (24). Longatto-Filho et al. (20) noted that LVDpt was correlated with CRC poor outcome markers, but not with significantly poor survival, while Matsumoto et al. (17) suggested that LVD was an independent prognostic factor of CRC. However, none of these studies analyzed the prognostic value of LVDpt or LVDit. In the present study, the survival curves demonstrated that both LVDit and LVDpt were associated with the overall survival of patients with CRC. The significance of LVDit and LVDpt for CRC prognosis was in agreement with that for gastric cancer (25).
There were several limitations in our study. Firstly, this was a retrospective study of post-surgical samples in a local medical institution and selection bias could not be avoided as the distribution of clinical characteristics of CRC patients. The results in this study needs to be validated in multicenter institutions prospectively. Secondly, further studies are required to elucidate the mechanisms underlying tumor associated lymphangiogenesis in CRC although it is true that lymphangiogenesis plays an important role in the progression of CRC.
In conclusion, our study showed that both intratumoral and peritumoral lymphangiogenesis occurs in CRC. Peritumoral lymphangiogenesis might have a more important role in lymph node metastasis compared with intratumoral lymphangiogenesis, while intratumoral lymphangiogenesis was found to be more correlated with tumor growth and histopathological differentiation. In addition, both high LVDit and LVDpt were predictive of poor prognosis in CRC. Considering the significance of intratumoral and peritumoral lymphangiogenesis contributing to CRC progression and prognosis, antilymphangiogenesis could be a valuable and reliable treatment for CRC.
Acknowledgments
Funding: The present study was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1038
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1038
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1038). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research was approved by the Ethics Committee of Peking University People’s Hospital (No. 2020PBH006-01), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Huber GF, Fritzsche FR, Züllig L, et al. Podoplanin expression correlates with sentinel lymph node metastasis in early squamous cell carcinomas of the oral cavity and oropharynx. Int J Cancer 2011;129:1404-9. [Crossref] [PubMed]
- Špirić Z, Erić M, Eri Ž, et al. Significantly high lymphatic vessel density in cutaneous metastasizing melanoma. Hippokratia 2015;19:210-5. [PubMed]
- Zhang S, Yi S, Zhang D, et al. Intratumoral and peritumoral lymphatic vessel density both correlate with lymph node metastasis in breast cancer. Sci Rep 2017;7:40364. [Crossref] [PubMed]
- Kostis G, Ioannis L, Helen K, et al. The expression of vascular endothelial growth factor-C correlates with lymphatic microvessel density and lymph node metastasis in prostate carcinoma: an immunohistochemical study. Urol Ann 2014;6:224-30. [Crossref] [PubMed]
- Rofstad EK, Huang R, Galappathi K, et al. Functional intratumoral lymphatics in patient-derived xenograft models of squamous cell carcinoma of the uterine cervix: implications for lymph node metastasis. Oncotarget 2016;7:56986-97. [Crossref] [PubMed]
- Liang P, Hong JW, Ubukata H, et al. Increased density and diameter of lymphatic microvessels correlate with lymph node metastasis in early stage invasive colorectal carcinoma. Virchows Arch 2006;448:570-5. [Crossref] [PubMed]
- Kaneko I, Tanaka S, Oka S, et al. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis Colon Rectum 2007;50:13-21. [Crossref] [PubMed]
- Chen Y, Yan J, Wang Z, et al. A meta-analysis of the relationship between lymphatic microvessel density and the survival of patient with colorectal cancer. Lymphology 2013;46:42-51. [PubMed]
- Ma Q, Dieterich LC, Detmar M. Multiple roles of lymphatic vessels in tumor progression. Curr Opin Immunol 2018;53:7-12. [Crossref] [PubMed]
- Wang Z, Wu J, Li G, et al. Lymphangiogenesis and biological behavior in pancreatic carcinoma and other pancreatic tumors. Mol Med Rep 2012;5:959-63. [Crossref] [PubMed]
- Rudno-Rudzinska J, Kielan W, Grzebieniak Z, et al. High density of peritumoral lymphatic vessels measured by D2-40/podoplanin and LYVE-1 expression in gastric cancer patients: an excellent prognostic indicator or a false friend? Gastric Cancer 2013;16:513-20. [Crossref] [PubMed]
- Choi Y, Park KJ, Ryu S, et al. Papillary thyroid carcinoma involving cervical neck lymph nodes: correlations with lymphangiogenesis and ultrasound features. Endocr J 2012;59:941-8. [Crossref] [PubMed]
- Garcia-Carracedo D, Rodrigo JP, Astudillo A, et al. Prognostic significance of lymphangiogenesis in pharyngolaryngeal carcinoma patients. BMC Cancer 2010;10:416. [Crossref] [PubMed]
- Omoto I, Matsumoto M, Okumura H, et al. Expression of vascular endothelial growth factor-C and vascular endothelial growth factor receptor-3 in esophageal squamous cell carcinoma. Oncol Lett 2014;7:1027-32. [Crossref] [PubMed]
- Saad RS, Kordunsky L, Liu YL, et al. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol 2006;19:1317-23. [Crossref] [PubMed]
- Matsumoto K, Nakayama Y, Inoue Y, et al. Lymphatic microvessel density is an independent prognostic factor in colorectal cancer. Dis Colon Rectum 2007;50:308-14. [Crossref] [PubMed]
- Barresi V, Reggiani-Bonetti L, Di Gregorio C, et al. Lymphatic vessel density and its prognostic value in stage I colorectal carcinoma. J Clin Pathol 2011;64:6-12. [Crossref] [PubMed]
- Du B, Yang ZY, Zhong XY, et al. Metastasis-associated protein 1 induces VEGF-C and facilitates lymphangiogenesis in colorectal cancer. World J Gastroenterol 2011;17:1219-26. [Crossref] [PubMed]
- Longatto-Filho A, Pinheiro C, Ferreira L, et al. Peritumoural, but not intratumoural, lymphatic vessel density and invasion correlate with colorectal carcinoma poor-outcome markers. Virchows Arch 2008;452:133-8. [Crossref] [PubMed]
- Royston DJ, Clasper S, Jackson DG. Immunohistochemical methods for measuring tissue lymphangiogenesis. Methods Mol Biol 2016;1430:35-48. [Crossref] [PubMed]
- Stacker SA, Williams SP, Karnezis T, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014;14:159-72. [Crossref] [PubMed]
- Hu X, Luo J. Heterogeneity of tumor lymphangiogenesis: progress and prospects. Cancer Sci 2018;109:3005-12. [Crossref] [PubMed]
- Gao J, Knutsen A, Arbman G, et al. Clinical and biological significance of angiogenesis and lymphangiogenesis in colorectal cancer. Dig Liver Dis 2009;41:116-22. [Crossref] [PubMed]
- Pak KH, Jo A, Choi HJ, et al. The different role of intratumoral and peritumoral lymphangiogenesis in gastric cancer progression and prognosis. BMC Cancer 2015;15:498-506. [Crossref] [PubMed]


