
Clinicopathological characteristics and treatment outcomes of cutaneous extranodal natural killer/T-cell lymphoma: a retrospective study in China
Introduction
According to the 2008 World Health Organization (WHO) classification of hematolymphoid tumors, extranodal natural killer/T-cell lymphoma (ENKTL) is a distinct type of lymphoma that is usually extranodal and characterized by vascular damage and destruction, necrosis, a cytotoxic phenotype, and Epstein–Barr virus (EBV) positivity. ENKTL is mostly reported in Asia, Mexico, and South America (1,2), and its treatment is a significant clinical challenge.
Depending on the site of the original lesion, ENKTL can be defined as nasal (ENKTL-N) or nasal type (ENKTL-NT). The former involves the upper aerodigestive tract, especially the nasal or sinonasal regions; and the latter usually occurs in the skin, gastrointestinal tract, testes, and soft tissues (3). The skin is the second most commonly affected site after the nasal cavity or nasopharynx (4), and can present as a primary or secondary manifestation. Initial presentation in the skin is known as primary cutaneous ENKTL-NT (PC-ENKTL-NT) and can occur with or without systemic symptoms (e.g., fever, night sweat, weight loss, and even hemophagocytic syndrome). Cases of advanced ENKTL-N with skin metastasis are termed secondary cutaneous ENKTL-N (SC-ENKTL-N) (5).
Cutaneous ENKTL has a short survival time and shows poor response to therapy; one study reported a median progression-free survival of 12 months and median overall survival (OS) of 29.0 months, with an 2-year OS rate of 48% and 12-month survival rate of 40% for advanced-stage disease. SC-ENKTL has a more aggressive clinical course and worse outcome than PC-ENKTL (5,6). However, these findings are based on a small number of studies and case reports, and the detailed clinical features and prognosis of cutaneous ENKTL have not been fully elucidated.
To this end, we carried out a retrospective analysis of a cohort of cutaneous ENKTL cases treated at our institution in order to identify the key prognostic factors and optimal treatment approaches. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1188).
Methods
Patients
A total of 47 cases of cutaneous ENKTL diagnosed at the Pathology Department, West China Hospital (Chengdu, China) from November 2000 to July 2016 were included in the analysis. Complete clinical and immunohistochemical data were available for 27 of the cases, while only the latter were available for the remaining 20 cases. Histologic diagnosis of the tumor was based on the WHO–European Organization for Research and Treatment of Cancer classification of cutaneous lymphomas (2005) (7) and WHO classification for tumors of hematopoietic and lymphoid tissues (2008) (8). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University (No. 2020731). Informed consent was waived in this retrospective study.
The inclusion criteria were as follows: (I) diagnosis of ENKTL based on biopsy slides; (II) diagnostic workup that included immunohistochemical detection of cluster of differentiation (CD)3 (cytoplasmic or surface), CD56, CD20, T-cell intracytoplasmic antigen 1, granzyme B, CD8, and Ki-67; in situ hybridization for EBV-encoded small RNA (EBER); and PCR detection of T-cell receptor gene rearrangement in a subset of cases using the BIOMED-2 system; and (III) central review by at least 2 expert pathologists.
Patient information included age; sex; clinical characteristics; lesion type and location; presence of B symptoms, performance status; complete blood analysis; serum lactate dehydrogenase (LDH) and β2 microglobulin levels; Ann Arbor stage; International Prognostic Index (IPI) score; bone marrow smear and biopsy results; neck/chest/abdominal/pelvic computed tomography (CT) or positron emission tomography/CT (PET/CT) scan findings; treatment type and evaluation of efficacy and toxicities; and length of follow-up. All patients had undergone staging evaluation that included a comprehensive physical examination and imaging. The International Society for Cutaneous Lymphomas and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer tumor–node–metastasis staging system for PC lymphoma other than mycosis fungoides and Sezary syndrome (9) was combined with Ann Arbor Staging Classification for SC-ENKTL to stage each case as follows: stage I, localized cutaneous disease (T1–2N0M0); stage II, localized disease with regional lymph node involvement (T1–2N1M0); stage III, disseminated disease without visceral organ involvement (T3 NX M0 or TX N2-3M0); and stage IV, disease with visceral organ involvement (TX NX M1). Responses to treatment were assessed using an adaption of Cheson’s standardized criteria (10).
Statistical analysis
OS was defined as the interval from the date of diagnosis to the date of death from any cause or the last follow-up visit. OS was estimated using the Kaplan-Meier product-limit method and compared with the log-rank test. Uni- and multivariate analyses were performed using the Cox proportional hazards regression model, and variables were selected using the enter method. A 2-sided P value <0.05 denoted statistical significance. SPSS v19.0 software (IBM, Armonk, NY, USA) was used for all analyses.
Results
Patient characteristics
The characteristics of patients are summarized in Tables 1,2. The mean age was 41 years (range, 7–72 years), with 2 subjects aged <7 years. The male-to-female ratio was 1.13:1.00.
Table 1
| Feature | Value, n [%] |
|---|---|
| Sex | |
| Male | 16 [59] |
| Female | 11 [41] |
| Age, years | |
| ≥60 | 7 [26] |
| <60 | 20 [74] |
| Mean age | 41 |
| Cutaneous involvement | |
| Primary | 21 [78] |
| Secondary | 6 [22] |
| Solitary | 3 [11] |
| Multiple | 24 [89] |
| Distribution | |
| Facial | 12 [44] |
| Trunk | 16 [59] |
| Limbs | 18 [67] |
| Genital | 5 [19] |
| Macroscopy | |
| Nodules | 18 [67] |
| Ulceration | 8 [30] |
| Erythema | 7 [26] |
| Ringworm-like lesions | 2 [7] |
| Swelling | 5 [19] |
| Extracutaneous findings in PC-ENKTL-NT | |
| Lymphadenopathy | 13 [62] |
| Upper respiratory and digestive tract | 6 [29] |
| Visceral | 7 [38] |
| Bone marrow involvement | 9 [33] |
| Clinical manifestation | |
| B symptoms | 14 [52] |
| Decreased WBC count | 14 [52] |
| Increased level of LDH | 20 [74] |
| Increased level of β2 | 13/14 [93] |
| EBV-DNA positive | 6/13 [46] |
| Stage | |
| I–II | 4 [15] |
| III–IV | 23 [85] |
PC-ENKTL-NT, primary cutaneous-extranodal natural killer/T-cell lymphoma-nasal type; WBC, white blood cell; LDH, lactic dehydrogenase; EBV, Epstein–Barr virus
Table 2
| No. | Sex/age | PC/SC | Cutaneous involvement† | Stage‡ | Extracutaneous | LDH§ | IPI¶ | Hem* | β2†† | Treatment | Evaluation | Statement | OS‡‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/38 | PC | Plaque, nodules, ulcer: trunk, limbs | IVB | Binasal, splenomegaly | 1 | 0 | U | 7 | LVD*[2] | PR | D+ | 4 |
| 2 | M/42 | PC | Plaque: limbs, trunk | IIIB | Paranasal, multi-LN | 1 | 1 | U | CLVP*[1] | PD | D+ | 1 | |
| 3 | M/36 | PC | Nodules: trunk | IVA | Multi-LN, bone marrow | 1 | 1 | U | CVAD*[1], IEC*[1], LVD*[1] | PD | D+ | 3 | |
| 4 | F/33 | PC | Nodules: limbs | IVB | Multi-LN, bone marrow | 1 | 1 | U | CDVP*[1] | PD | D+ | 2 | |
| 5 | F/22 | PC | Swelling: eyelid | IVB | Multi-LN, splenomegaly, bone marrow | 1 | 1 | U | CHOP*[1] | SD | D+ | 1 | |
| 6 | F/71 | PC | Lesions, blisters: lip | IVB | Lung | 0 | 1 | N | BSC | PD | D+ | 2 | |
| 7 | M/21 | PC | Swelling, nodules: eyelid, trunk | IVB | Splenomegaly | 1 | 1 | U | BSC | PD | D+ | 3 | |
| 8 | F/73 | PC | Nodules: leg, jaw | IIIA | None | 1 | 0 | N | 4 | LVP*[4] | SD | D+ | 10 |
| 9 | F/19 | SC | Nodules: back, leg | IVA | Bone marrow | 1 | 1 | N | CHOP*[1] LVD | PD | D+ | 6 | |
| 10 | M/60 | PC | Plaque: trunk, limbs | IVA | Splenomegaly | 1 | 1 | U | BSC | PD | D+ | 2 | |
| 11 | F/19 | PC | Nodules, ulcer: leg, genital | IVA | Multi-LN, splenomegaly | 1 | 1 | N | BSC | PD | D+ | 3 | |
| 12 | M/55 | PC | Nodules: eyelid, trunk | IVB | Inguinal LNs, bone marrow | 1 | 1 | U | SMILE*[3] | CR | Do | 2 | |
| 13 | M/60 | PC | Nodules, ulcer: trunk, limbs, genital | IVB | Bi-inguinal LNs, paranasal sinus | 1 | 1 | N | Prednisone | SD | D+ | 4 | |
| 14 | M/69 | PC | Nodules: limbs | IVB | Multi-LN, nasal, bone marrow | 1 | 1 | U | 3 | GLECD*[2] Radiation | PR | D+ | 5 |
| 15 | M/55 | SC | Nodules: limbs, trunk, genital | IIIA | None | 0 | 1 | N | 3 | GLECD*[3] | PR | D+ | 7 |
| 16 | M/62 | PC | Swelling: trunk | IVB | Liver, splenomegaly, bone marrow | 1 | 1 | U | 9 | GLECD*[1] | PR | D+ | 2 |
| 17 | M/40 | SC | Nodules, swelling: eyelid | IIA | Left jaw LN | 1 | 0 | N | 4 | VDLP [san] | CR | A+ | 32 |
| 18 | F/55 | PC | Plaque, ulcer: trunk | IIA | Bicervical LNs | 0 | 0 | N | 3 | CEOP*[1] | PD | A+ | 30 |
| 19 | F/34 | PC | Plaque, blisters: limbs, trunk | IVA | Nasal and paranasal cavities | 1 | 0 | U | GLECD*[2] | PR | D+ | 4 | |
| 20 | F/16 | PC | Nodules, ulcer: Trunk, limbs | IIIA | None | 0 | 0 | U | 4 | CHOP | PD | D+ | 6 |
| 21 | M/38 | SC | Ulcer: face | IVB | Nasopharynx, amygdala | 0 | 0 | N | CHOP*[2]; radiation | PR/PD | D+ | 8 | |
| 22 | M/27 | PC | Nodules, papule: limbs, trunk | IVB | Bicervical LNs, bone marrow | 1 | 1 | N | 6 | BSC | PD | D+ | 6 |
| 23 | M/50 | PC | Lesions: legs, trunk | IIIA | None | 1 | 0 | N | 3 | VDLP*[san] | CR | A+ | 24 |
| 24 | M/61 | PC | Plaque, ulcer: left arm | IIA | Left axilla LN | 0 | 0 | N | 3 | VDLP*[4] | CR | A+ | 22 |
| 25 | M/56 | SC | Nodules, ulcer: face | IIA | None | 0 | 0 | N | 1 | VDLP*[san] | CR | A+ | 20 |
| 26 | F/45 | SC | Nodules, ulcer: limbs, trunk | IIIB | Bicervical, axilla LNs | 1 | 1 | U | 2 | Radiation, CHOP*[2] | PD | D+ | 2 |
| 27 | F/62 | PC | Nodules: eyelid | IVB | Lung, bone marrow | 1 | 1 | U | 5 | BSC | PD | D+ | 2 |
†, Presentation and area of skin;. ‡, Stage I, localized cutaneous disease (T1–2N0M0); stage II, localized disease with regional LN involvement (T1–2N1M0); stage III, disseminated disease without visceral organ involvement (T3 NX M0 or TX N2-3M0); stage IV, disease with visceral organ involvement (TX NX M1); A, without B symptoms; B, with B symptoms;. §,IU/lL; 0=normal; 1=high; cutoff: 250;. ¶, 0=low 0–2; 1= high 3–5;. *, U = abnormal; N = normal;. ††, mg/L; cutoff =2;. ‡‡, Months. A+, alive; BSC, best supportive care; β2, beta 2 microglobulin; CEOP, cyclophosphamide, etoposide, vincristine, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CLVD, cyclophosphamide, L-asparaginase, vincristine, and dexamethasone; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; D+, died of disease; Do, died of treatment-related complications; ENKTL, extranodal natural killer/T-cell lymphoma; F, female; GLECD, gemcitabine, L-asparaginase, etoposide, cyclophosphamide, and prednisone; Hem, hemogram; IPI, International Prognostic Index; IEC, ifosfamide, etoposide, and carboplatin; LDH, lactate dehydrogenase; LN, lymph node; LVD, L-asparaginase, vincristine, and dexamethasone; LVP, L-asparaginase, vincristine, and prednisolone; M, male; PC, primary cutaneous; san, sandwich; SC, secondary cutaneous; SMILE, dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide; VDLP, L-asparaginase, etoposide, prednisone, cisplatin chemotherapy with radiotherapy.
Immunophenotype and genotype
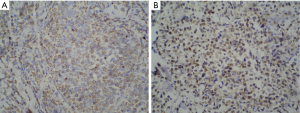
The results of immunohistochemical analysis of tumor tissue sections are shown in Table 3. In all cases, tumor cells were negative for the B-cell differentiation marker CD20; 37/42 cases were positive for CD56, 34/36 for cytoplasmic CD3 (CD3ε), and 34/40 for granzyme B. Ki-67 staining was performed in 27/47 cases and the mean positive rate was 68%. Additionally, 16/22 cases were positive for CD3 (Figure 1) and 36/40 for EBER; and 13/27 cases were partially positive for CD30.
Table 3
| CD20 | CD56 | CD3ε | CD3 | Granzyme B | EBER | CD30 | |
|---|---|---|---|---|---|---|---|
| Positive, n [%] | 0 [0] | 37 [88] | 34 [94] | 16 [73] | 34 [85] | 36 [90] | 13 [48] |
| Negative, n [%] | 47 [100] | 5 [12] | 2 [6] | 6 [27] | 6 [15] | 4 [10] | 14 [52] |
| Unknown, n [%] | 0 | 5 | 11 | 25 | 7 | 7 | 20 |
CD3ε, cytoplasmic CD3; EBER, EBV-encoded small RNA.
Clinical features
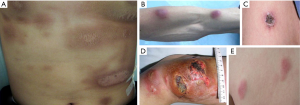
Lesions were predominantly located on the extremities (18/27, 67%), trunk (16/27, 59%), face (12/27, 44%), and genitals (5/27, 19%). In total, 24/27 patients 89% showed skin involvement at multiple locations, which presented as erythematous, nodular, swollen, or ringworm-like lesions accompanied by painless ulceration (Figure 2). Primary skin involvement was reported in 21 patients. However, in 6 cases, lesions initially occurred in the nasal region before progressing to the skin (several months to a few years later). In 4 of these patients, skin manifestations emerged within 6 months of ENKTL-N diagnosis during treatment. Two patients were diagnosed with early ENKTL-N and received nasal radiotherapy, but experienced skin recurrence after >2 years of complete remission (CR). Among patients with PC-ENKTL-NT, 78% had extracutaneous lesions detected by CT or PET/CT scan: 62% (13/21 patients) had lymphadenopathy in various parts of the body, 29% (6/21 patients) had upper aerodigestive tract lesions, and 38% (8/21 patients) showed visceral involvement. Four patients had stage I/II disease and 23 had stage III/IV disease. Fourteen patients developed systemic symptoms, with fever as the main complaint. Leukopenia was detected in 14 patients upon admission. One-third of patients (9/27) had bone marrow involvement with or without a decrease in platelet number and hemoglobin level. Of the patients who were tested, 74% (20/27) showed increased LDH level, which was ≥500 IU/L in 10 cases. β2 Microglobulin level was elevated in the majority of patients (93%; 13/14 patients) (Tables 1,2).
Treatment and evaluation
Clinical data were available for 27 cutaneous ENKTL patients. In terms of treatment, 14 patients received chemotherapy alone; 6 received chemotherapy and involved-field radiotherapy; 3 with bacterial infection and 1 with serious hemophagocytic syndrome received supportive care only; and the 3 remaining patients declined treatment for religious reasons. In the absence of standard chemotherapeutic agents, different treatment regimens were selected by physicians based on tumor stage and IPI score, but most included L-asparaginase (L-asp)—i.e., L-asp, vincristine, and prednisolone (LVP); dexamethasone, methotrexate, ifosfamide, L-asp, and etoposide (SMILE); gemcitabine, L-asp, etoposide, cyclophosphamide, and prednisolone (GLECD); etoposide, cisplatin, L-asp, and prednisone (VDLP). Only 2 patients with PC-ENKTL-NT received cyclophosphamide, vincristine, doxorubicin, and dexamethasone (CHOP) as initial therapy (Table 2).
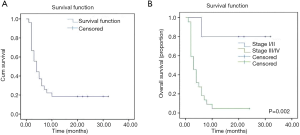
Treatment outcomes were generally poor, with only 9/20 patients showing a sustained response; these 9 patients received chemotherapy with L-asp, and 4 achieved CR. A CR was observed in 2/14 (14%) patients receiving chemotherapy only and 3/6 (50%) patients receiving chemotherapy plus radiotherapy. Figure 3A shows the survival of the 27 patients. The median duration of follow-up was 4.0 months (range, 1.0–32.0 months), and 22 patients (81.5%) died. The median survival time was 4.0 months (95% CI: 2.3–5.7 months), and mean survival time was 9.1 months (95% CI: 4.9–13.3 months). In the 5 surviving patients, survival time was >20 months; all 5 cases presented with skin lesions and received treatment that included L-asp. Figure 3B compares the OS between the 4 stage I/II patients and 23 stage III/IV patients with nasal, visceral, or bone marrow involvement. Patients with localized disease (early stage I/II) had a better outcome, with a mean survival time >26 months (95% CI: 17.68–35.92 months). Stage III/IV patients had a very poor outcome (OS: 4.7 months; 95% CI: 1.01–2.73 months). Survival rates differed significantly between the 2 groups (log-rank test, χ2=9.97; P=0.002). All 4 patients who were treated with the VDLP regimen achieved CR. Notably, a female with stage IIA disease (Patient 18) declined further therapy after 1 cycle of CEOP (cyclophosphamide, etoposide, vincristine, and prednisone) chemotherapy, citing religious beliefs. Nonetheless, her skin lesions completely disappeared 3 months after discharge. We reviewed the pathologic features of this case and confirmed that there was no misdiagnosis. It is unclear whether autoimmunity or other factors exerted an anti-tumor effect.
Uni- and multivariate analyses were carried out using the Cox proportional hazards model with the following parameters: age, sex, disease subtype (PC-/SC-ENKTL), extracutaneous findings, stage, B symptoms, LDH, hemoglobin, Ki-67, EBV, β2 microglobulin, and inclusion of L-asp in the treatment regimen (Table 4). We found that the presence of B symptoms, advanced stage, elevated LDH level, abnormal hemoglobin level, and high IPI were independent prognostic factors for poor survival. A multivariate analysis was performed with the statistically significant parameters from the univariate analysis (i.e., B symptoms, stage, LDH, hemoglobin, and IPI). The results showed that abnormal hemoglobin level (hazard ratio [HR]: 7.70; 95% CI: 2.18–27.27; P=0.002) and IPI score (HR: 8.93; 95% CI: 2.36–33.78; P=0.001) were significantly associated with poor prognosis, while B symptoms (HR: 0.762; 95% CI: 0.208–2.800; P=0.682), stage (HR: 1.409; 95% CI: 0.880–2.255; P=0.072), and LDH (HR: 1.665; 95% CI: 0.402–6.897; P=0.482) were not associated with survival.
Table 4
| Survival | Covariate | HR | 95% CI | P |
|---|---|---|---|---|
| Univariate analysis for each covariate in the entire cohort (N=27) | B symptoms (positive vs. negative) | 4.26 | 1.78–13.07 | 0.002** |
| Stage (I/II vs. III/IV) | 6.4 | 1.79–22.92 | 0.004** | |
| LDH value (normal vs. high) | 4..26 | 1.39–13.09 | 0.011* | |
| Hemogram (normal vs. abnormal) | 6.71 | 2.23–20.18 | 0.001** | |
| IPI (high: 3–5 vs. 0–2) | 8.33 | 2.33–29.79 | 0.001** | |
| Multivariate analyses with the Cox proportional hazards model (N=27) | Hemogram (normal vs. abnormal) | 7.7 | 2.18–27.27 | 0.002** |
| IPI (high: 3–5 vs. 0–2) | 8.93 | 2.36–33.78 | 0.001** |
*, P<0.05; **, P<0.01.
Discussion
Most patients in the study were middle-aged (40–60 years; average age: 41 years) and the sex ratio was slightly skewed toward males (male-to-female ratio =1.13:1.00), which is similar to the demographic profile of the cohort in a previous study (6). Lesions were predominantly located on the extremities (67%), trunk (59%), and face (44%), although genitals (19%) were also frequently involved. As in other reports, lesions mainly presented as single or multiple nodules, erythema and rash, or painless necrotic ulcers, and PC-ENKTL (77%) was the most common subtype of cutaneous ENKTL (5,6).
All cases were negative for the B-cell differentiation antigen CD20 and in most patients, malignant cells were positive for the T cell differentiation antigen CD3ε and natural killer (NK) cell-associated marker CD56. However, some variations in immunohistochemical phenotype were observed in our cohort: 16/22 cases (73%) expressed surface CD3, which is markedly higher than the rate suggested by reference diagnostic criteria for cutaneous cases that typically show CD56, EBER, and CD3ε positivity and an absence of surface CD3 (11,12). It is possible that the variable expression of CD3 in nasal vs cutaneous ENKTL reflects a difference in tumor origin or pathogenesis. Indeed, differences between these 2 subtypes of ENKTL are currently under investigation. Experiments using ENKTL cell lines have revealed the existence of at least 2 lineages—namely, CD3−/CD3ε+/CD20−/CD56+ NK cells and CD3+/CD3ε+/CD20−/CD56+ γδ T cells (13). In a study of upper aerodigestive tract ENKTL of T cell vs. NK cell origin, the former showed a trend for longer survival time (14). A comparative analysis of genomic alterations in 4 patients with PC-ENKTL-NT and 1 patient with SC-ENKTL-N by array-based genomic hybridization showed distinct patterns of chromosomal gain and loss (5), which could be useful for distinguishing subsets of ENKTL with cutaneous involvement. However, whether differences in CD3 expression are related to the degree of tumor differentiation requires further experimental verification. Additionally, 5 patients (5/42) with ENKTL were negative for CD56 expression. Compared to ENKTL confined to the nasal cavity, patients with extranasal upper aerodigestive tract involvement were more likely to be CD56-negative (15); and 20% of true NK cell tumors with germline T cell receptor γ gene were CD56-negative, while 45% of T cell-derived tumors lacked CD56 expression. In cases that are negative for CD56, analysis of cytotoxic marker expression and EBER status are required for the diagnosis of ENKTL (16). The significance of these biological differences for response to treatment and prognosis warrants further investigation.
Consistent with previous reports, systemic chemotherapy was the first-choice treatment in our cohort. Most of the regimens (LVP, SMILE, GLECD, and VDLP) contained L-asp. Chemotherapy was effective in fewer than half (9/20) of the patients. Among the 9 responders, 4 had achieved CR at the time of treatment completion (Patients 18, 23, 24, and 25). SMILE was administered in only 1 patient (Patient 12) who had advanced-stage disease with bone marrow involvement; CR was observed after 3 cycles of treatment. However, the patient died due to treatment-related complications. The same outcome was reported in a small proportion of cases in a phase I/II study of this regimen (17,18). SMILE was associated with an overall response rate of 80%, even including stage III–IV non-nasal patients (19). However, severe bone marrow suppression and infection should be taken into account (19,20). Consistent with previous reports supporting the limited use of the CHOP regimen in ENKTL, anthracycline-based chemotherapy (Patients 5, 9, 20, and 21) resulted in a poor outcome (21). For the 11 patients in whom initial chemotherapy was ineffective, second-line treatment options were limited. In recent years, L-IMEP (L-asp, ifosfamide, methotrexate, etoposide and prednisolone) (22), VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone) (23), LVP (24), GELOX (gemcitabine, L-asp, and oxaliplatin) (25), and DDGP (cisplatin, dexamethasone gemcitabine, and pegaspargase) (26) regimens have produced encouraging responses in both early- and advanced-stage patients.
More patients achieved CR with chemoradiotherapy than with chemotherapy alone in our study. In a global prospective cohort study of 166 patients diagnosed with ENKTL in which response to therapy was evaluable, patients with stage I or II disease who were treated with chemotherapy in combination with radiotherapy (79%) achieved a higher CR rate than those treated with chemotherapy (13%) or radiotherapy (25%) only (27). In a retrospective analysis of 20 patients with stage I/II cutaneous ENKTL, radiotherapy (n=5) yielded a higher CR rate than chemotherapy (n=15) (80% vs. 40%, respectively) (4). However, as radiation therapy is associated with systemic failure (25%) its utility for the treatment of non-nasal cases remains unclear. As a core component of the recommended first-line treatment, radiotherapy should be promptly administered to patients with nasal skin lesions or localized disease (21,28).
As shown in Figure 3, most stage III/IV patients had very poor outcome, in agreement with earlier studies (4,29). Notably, in a stage IIA patient (patient 18) whose disease was not controlled after 1 cycle of chemotherapy, the lesions completely disappeared after 3 months without further treatment. The role of immune factors in this highly aggressive tumor type merits further attention. In fact, immune checkpoint inhibitors have yielded promising results for the treatment of ENKTL (30).
We used the forward conditional Cox regression model (19) in our analysis. The multivariate analysis identified 2 factors—i.e., high IPI score and abnormal hemoglobin level—as independent prognostic factors. These results were expected given that prognostic scores have been re-evaluated since the introduction of intensive chemotherapy regimens such as SMILE. Unlike the Korean prognostic score (19), the IPI score was found to be significant, especially in non-nasal cases. In the univariate analysis, LDH level and Ann Arbor stage were identified as independent prognostic factors for poor survival, and were likely highly correlated with IPI score. However, the score also includes age, number of extranodal lesions, and Eastern Cooperative Oncology Group score. Therefore, a more accurate scoring model that accurately reflects prognosis is needed. Two prognostic models—namely, the Prognostic Index of Natural Killer Cell Lymphoma (PINK) (including age, stage, distant lymph node involvement, and non-nasal type) and PINK-E (PINK with EBV-DNA added) have recently been developed for use in patients with ENKTL receiving non-anthracycline-based therapy (31). Cases of bone marrow involvement are associated with confounding factors, especially in patients exhibiting hemophagocytosis and hepatosplenomegaly. Lymphocyte infiltration in the bone marrow was detected in our cohort; however, the EBER phenotype was absent. Therefore, in some cases a diagnosis of bone marrow involvement requires a more comprehensive examination. An abnormal peripheral hemogram is another potential prognostic factor in cutaneous ENKTL.
Conclusions
The results of this study show that cutaneous ENKTL differs from primary nasal ENKTL in terms of clinical and biological characteristics. PC-ENKTL-NT accounts for the majority of cutaneous cases. Localized disease was associated with better outcome than disseminated disease with or without involvement of viscera and other organs. L-asp-containing chemotherapy—especially the VDLP and SMILE regimens—yielded good response rates. However, further studies are needed to clarify the molecular basis of cutaneous ENKTL and establish a standardized treatment for this disease.
Acknowledgments
Funding: This work was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1188
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1188
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-20-1188
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1188). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University (No. 2020731). Informed consent was waived in this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Linke-Serinsöz E, Fend F, Quintanilla-Martinez L. Human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV) related lymphomas, pathology view point. Semin Diagn Pathol 2017;34:352-63. [Crossref] [PubMed]
- Laurini JA, Perry AM, Boilesen E, et al. Classification of non-Hodgkin lymphoma in Central and South America: A review of 1028 cases. Blood 2012;120:4795-801. [Crossref] [PubMed]
- Tse E, Kwong TL. Diagnosis and management of extranodal NK/T cell lymphoma nasal type. Expert Rev Hematol 2016;9:861-71. [Crossref] [PubMed]
- Ahn HK, Suh C, Chuang SS, et al. Extranodal natural killer/T-cell lymphoma from skin or soft tissue: suggestion of treatment from multinational retrospective analysis. Ann Oncol 2012;23:2703-7. [Crossref] [PubMed]
- Berti E, Recalcati S, Girgenti V, et al. Cutaneous extranodal NK/T-cell lymphoma: A clinicopathologic study of 5 patients with array-based comparative genomic hybridization. Blood 2010;116:165-70. [Crossref] [PubMed]
- Lee WJ, Jung JM, Won CH, et al. Cutaneous extranodal natural killer/T-cell lymphoma: A comparative clinicohistopathologic and survival outcome analysis of 45 cases according to the primary tumor site. J Am Acad Dermatol 2014;70:1002-9. [Crossref] [PubMed]
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768-85. [Crossref] [PubMed]
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011;117:5019-32. [Crossref] [PubMed]
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110:479-84. [Crossref] [PubMed]
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86. [Crossref] [PubMed]
- Chan JK, Tsang WY, Ng CS. Clarification of CD3 immunoreactivity in nasal T/natural killer cell lymphomas: The neoplastic cells are often CD3 epsilon+. Blood 1996;87:839-41. [Crossref] [PubMed]
- Jaffe ES, Krenacs L, Raffeld M. Classification of cytotoxic T-cell and natural killer cell lymphomas. Semin Hematol 2003;40:175-84. [Crossref] [PubMed]
- Nagata H, Konno A, Kimura N, et al. Characterization of novel natural killer (NK)-cell and γδ T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein-Barr virus. Blood 2001;97:708-13. [Crossref] [PubMed]
- Pongpruttipan T, Sukpanichnant S, Assannsen T, et al. Extanodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: A comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol 2012;36:481-99. [Crossref] [PubMed]
- Li YX, Wang H, Feng XL, et al. Immunophenotypic characteristics and clinical relevance of CD56+ and CD56− extranodal nasal-type natural killer/T-cell lymphoma. Leuk Lymphoma 2011;52:417-24. [Crossref] [PubMed]
- Ng SB, Lai KW, Murugaya S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: A clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol 2004;17:1097-107. [Crossref] [PubMed]
- Yamaguchi M, Suzuki R, Kwong YL, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced‐stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci 2008;99:1016-20. [Crossref] [PubMed]
- Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 2011;29:4410-6. [Crossref] [PubMed]
- Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer (NK)/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 2012;120:2973-80. [Crossref] [PubMed]
- Kim SJ, Park S, Kang ES, et al. Induction treatment with SMILE and consolidation with autologous stem cell transplantation for newly diagnosed stage IV extranodal natural killer/T-cell lymphoma patients. Ann Hematol 2015;94:71-8. [Crossref] [PubMed]
- Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood 2018;131:2528-40. [Crossref] [PubMed]
- Kim M, Kim TM, Kim KH, et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus L-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL). Ann Hematol 2015;94:437-44. [Crossref] [PubMed]
- Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol 2009;27:6027-32. [Crossref] [PubMed]
- Zhang L, Jiang M, Xie L, et al. Five-year analysis from phase 2 trial of “sandwich” chemoradiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer Med 2016;5:33-40. [Crossref] [PubMed]
- Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer 2013;119:348-55. [Crossref] [PubMed]
- Zhao Q, Fan S, Chang Y, et al. Clinical efficacy of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) in the initial treatment of advanced stage (stage III-IV) extranodal NK/T-cell lymphoma, and its correlation with Epstein-Barr virus. Cancer Manag Res 2019;11:3555-64. [Crossref] [PubMed]
- Fox CP, Civallero M, Ko YH, et al. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: A prospective cohort study from the International T-cell Project. Lancet Haematol 2020;7:e284-94. [Crossref] [PubMed]
- Moncada B, Sobrevilla-Ondarza S, Md JD. Radiotherapy supports a better outcome than chemotherapy in cutaneous natural killer (NK)/T cell lymphoma nasal type. Int J Dermatol 2013;52:1276-7. [Crossref] [PubMed]
- Yu JB, Zuo Z, Tang Y, et al. Extranodal nasal-type natural killer/T-cell lymphoma of the skin: A clinicopathologic study of 16 cases in China. Hum Pathol 2009;40:807-16. [Crossref] [PubMed]
- Kwong YL, Chan TS, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing L-asparaginase. Blood 2017;129:2437-42. [Crossref] [PubMed]
- Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: A multicentre, retrospective analysis. Lancet Oncol 2016;17:389-400. [Crossref] [PubMed]


