
Long non-coding RNA Linc00261 as a novel potential diagnostic and prognostic biomarker for gallbladder cancer
Introduction
Gallbladder carcinoma (GBC) is the most common cancer of the bile duct, which accounts for 1.2% of all global cancer diagnoses and 1.7% of all cancer deaths (1,2). This carcinoma is infrequent in developed countries but more common in some developing countries. The incidence of GBC in female patients was significantly higher than that in male (3,4). Compared with other malignant bile duct tumors, GBC has a poorer prognosis, as the 5-year survival rate for patients with the disease was between 5% and 13% with a median survival of only about 6 months (5). Despite advances in the modalities used for GBC diagnosis and treatment, the clinical outcomes of GBC have not significantly improved because there are no specific symptoms in patients with early-stage carcinoma of the gallbladder and its diagnosis is often delayed (6,7). A surgical resection is currently the only effective treatment for GBC, but aggressive surgical approaches may result in high surgical morbidity and mortality rates (8,9). Therefore, studies aiming to elucidate the molecular mechanisms mediating GBC initiation and progression, improve the methods of early diagnosis, are urgently needed.
Long non-coding RNAs (lncRNAs) is a kind of extensive transcription with RNA transcripts longer than 200 nucleotides (nt) that lack of coding protein function(10). It has been proved that lncRNAs may involve in various physiological and pathological processes such as cell proliferation metastasis and carcinogenesis by regulating transcription, translation as well as post-transcriptional and post-translational processing (11,12).
Long intergenic non-protein coding RNA 261 (Linc00261) is a lncRNA, shown to play key roles in the tumor suppression (13-15). It has been revealed that level of Linc00261expression was significantly lower in choriocarcinoma tissues and cell lines (14). Linc00261 overexpression in choriocarcinoma or hepatocellular carcinoma cell lines would decrease cell proliferation and inhibit cell invasion in vitro (14,16). However, the expressional level and functional role of Linc00261 in GBC has remained largely unknown. We hypothesized that Linc00261, as an inhibitor of human tumorigenesis, may also play important role in GBC development and progression.
This research aimed to investigate the function of Linc00261 associated with GBC development, which may enable clinicians and researchers to identify GBC-specific diagnostic biomarkers and to develop therapies capable of preventing cancer invasion and metastases.
Methods
Human sample
This study was approved by the ethical committee of the First People’s Hospital in Yunnan Province. A total of 100 patients diagnosed with GBC in Hepatopancreatobiliary Surgery Department of the First People’s Hospital in Yunnan Province were admitted to this study. Tumor tissues from 100 patients and their adjacent normal tissues (3 cm apart to the focus) were collected in sterile tube and stored in liquid nitrogen immediately. Serum Linc00261 levels in 50 GBC patients and 30 healthy individuals were measured and compared. 20 GBC patients’ blood samples were collected 1 week after surgery. Blood sample (2 mL) was placed in a vacutainer tube containing anticoagulant for isolation of serum. Supernatant was collected after centrifugation of blood. All simples were stored in liquid nitrogen. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the First People’s Hospital of Yunnan Province (approval number: KY-E-2017-1-20). All patients showed their full intention to participate in this study, and written consent from each patient was collected.
Cell culture
Three GBC cell lines including EH-GB1 (RRID: CVCL_IU73), SGC-996 (RRID: CVCL_M737), GBC-SD (RRID: CVCL_6903), NOZ NOZ (RRID: CVCL_3079) and the human intrahepatic biliary epithelial cell line HIBEC were obtained from the Hepatopancreatobiliary Surgery Department of the First People’s Hospital. All of these cells were cultured in the Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA) supplied with 10% fetal bovine serum (FBS; Gibco, USA) at a 5% CO2 atmosphere in a 37 °C incubator. After 24 h of culture, cells were digested and passage or used to extract RNA.
RNA extraction and qRT-PCR
Total RNA was extracted from human tissues, cultured cell lines and serum by TRIzol reagent (TaKaRa, China) with a concentration of 1 ml for each well in six-well plates. Cell lines and serum have been centrifuged and removed supernatant before extraction. cDNA was reversely transcribed by Transcriptor First Strand cDNA Synthesis Kit (TaKaRa). RT-PCR was then performed using a Power SYBR Green PCR Master Mix (Applied Biosystems, Life Technologies) and Bio-Rad CFX96 real-time PCR System. Gapdh was included as an internal control. The primers of Linc00261 and Gapdh in previous report (17) were used.
The sequence of Linc00261 primers are 5'-GTCAGAAGGAAAGGCCGTGA-3' (sense) and 3'-TGAGCCGAGATGAACAGGTG-5'(antisense). The sequence of Gapdh primers are 5'-GCTCTCTGCTCCTCCTGTTC-3' (sense) and 5'-ACGACCAAATCCGTTGACTC-3' (antisense).
Statistical analysis
All statistical data were analyzed by SPSS 19.0 version software. The relationship between the expressions of Linc00261 in GBC with the clinicopathological features of the patients was estimated by the chi-square test. The differences in overall survival (OS) curves and the progress free survival (PFS) rate were compared using the Kaplan-Meier, and the independent prognostic factors of the patients (including gender, age, tumor size, TNM stage, differentiation, Lymph node metastasis) were analyzed by Cox model. The diagnosis efficacy of using the serum Linc00261 level to identify the GBC has been investigated by ROC curve analysis. A P value <0.05 (bilateral test) was considered statistically significant in all the examinations. Statistically significant values for <0.05, <0.01, and <0.001 are indicated by single (*), double (**) and triple (***) asterisk, respectively.
Results
Linc00261 is significantly down-regulated in GBC tissues and cell lines
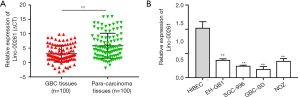
First, we examined the Linc00261 expression in 100 paired clinical GBC tissues and para-carcinoma tissues by qRT-PCR. As shown in Figure 1A, the expression of Linc00261 was significantly down-regulated in GBC tissues compared with adjacent normal tissues (P<0.01). We also compared the level of Linc00261 between normal human intrahepatic biliary epithelial cell line HIBEC and GBC cell lines (SGC-996, GBC-SD and NOZ) using qRT-PCR (Figure 1B). The results showed that GBC cell lines expressed lower Linc00261 compared with normal.
The expression of Linc00261 is associated with clinicopathological characteristics of GBC patients
To elucidate the potential function of Linc00261 in GBC, we analyzed the relationship between the Linc00261 expression level and the clinicopathological factors of the GBC patients. As shown in Table 1, the findings revealed that the lower level of Linc00261 expression was correlated with large tumor size (P<0.0001), late TNM stage (P=0.008), negative liver metastasis (P=0.027) and high differentiated degree (P=0.017). But gender and age of patients has no significant association with Linc00261 expression (P>0.05, respectively).
Table 1
| Clinicopathological characteristics | Total | Low expression | High expression | χ2 | P value |
|---|---|---|---|---|---|
| Gender | 0.360 | 0.548 | |||
| Male | 51 | 27 | 24 | ||
| Female | 49 | 23 | 26 | ||
| Age, years | 1.004 | 0.316 | |||
| ≤50 | 53 | 24 | 29 | ||
| >50 | 47 | 26 | 21 | ||
| Tumor size | 16.000 | <0.0001 | |||
| T1 | 24 | 8 | 26 | ||
| T2 | 19 | 7 | 12 | ||
| T3 | 20 | 12 | 8 | ||
| T4 | 27 | 23 | 4 | ||
| Differentiation | 8.174 | 0.017 | |||
| High | 34 | 20 | 10 | ||
| Moderate | 33 | 18 | 15 | ||
| Poor | 33 | 12 | 25 | ||
| Lymph node metastasis | 4.889 | 0.027 | |||
| Positive | 61 | 33 | 22 | ||
| Negative | 39 | 17 | 28 | ||
| TMN stages | 11.934 | 0.008 | |||
| I | 21 | 5 | 16 | ||
| II | 25 | 10 | 15 | ||
| III | 25 | 15 | 10 | ||
| IV | 29 | 20 | 9 |
The median value of the Linc00261 level in tissues of 100 GBC patients was considered as the cut-off value of all samples. 50 patients with Linc00261 expression lower than the cut-off value was classified into low expression group. Other 50 patients with higher expression were in high expression group. The relationship between expression and features of patients has been analyzed by χ2 test.
Low Linc00261 expression predicts poor prognosis in GBC
We have indicated that the expression of Linc00261 has correlation with the clinicopathological features of GBC. To further determine the prognostic effect of Linc00261 expression in GBC patients, we conducted a Kaplan-Meier survival analysis as shown in Figure 2. For the patients with low Linc00261 expression, Kaplan-Meier analysis revealed that they had significantly worse outcomes in terms of overall survival (OS, P=0.0188) and progression-free survival (PFS, P=0.0029) (Figure 2A,B).

High Linc00261 expression is an independent prognostic factor for high OS and PFS rate in GBC
Moreover, to evaluate whether Linc00261 expression was an independent prognostic factor for survival in GBC, univariate analyses by Cox model were conducted. The univariate analysis of OS shown in Table 2 describes the significant prognostic factors for OS including the level of Linc00261 expression (P=0.005), lymph node metastasis (P=0.032) and TNM stage (P=0.033). Table 3 demonstrated the results of univariate analysis of PFS. The significant prognostic factors for PFS included the Linc00261 expression (P<0.001), lymph node metastasis (P=0.005) and TNM stage (P=0.004) as well. However, other factors including gender, age, tumor size and differentiated degree had no significant effect both on OS and PFS (P>0.05). It is noteworthy that the low expression of Linc00261 is the most significant unfavorable factor for OS and PFS rate compared with the other candidate factors.
Table 2
| OS | B | SE | Wald | df | P value | Exp(B) | 95% Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Upper limit | Lower limit | |||||||
| Linc00261 | −0.745 | 0.264 | 7.941 | 1 | 0.005 | 0.475 | 0.283 | 0.797 |
| Gender | −0.046 | 0.228 | 0.041 | 1 | 0.840 | 0.955 | 0.611 | 1.492 |
| Age | 0.409 | 0.248 | 2.714 | 1 | 0.099 | 1.506 | 0.925 | 2.450 |
| Tumor size | 0.108 | 0.312 | 1.335 | 3 | 0.721 | 1.114 | 0.604 | 2.055 |
| Differentiation | −0.025 | 0.277 | 0.973 | 2 | 0.615 | 0.975 | 0.567 | 1.678 |
| Lymph node metastasis | 0.670 | 0.311 | 4.625 | 1 | 0.032 | 1.954 | 1.061 | 3.597 |
| TMN stage | −1.008 | 0.464 | 8.743 | 3 | 0.033 | 0.365 | 0.147 | 0.907 |
Univariate analyses of OS by Cox model were conducted to evaluate the potential prognostic factor in GBC. The candidate factors included the level of Linc00261 expression, gender, age, tumor size and differentiated degree, lymph node metastasis and TNM stage. B means regression coefficient. SE means standard error. df means degree of freedom.
Table 3
| PFS | B | SE | Wald | df | P value | Exp(B) | 95% Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Upper limit | Lower limit | |||||||
| Linc00261 | −1.183 | 0.283 | 17.520 | 1 | 0.000 | 0.306 | 0.176 | 0.533 |
| Gender | −0.029 | 0.229 | 0.017 | 1 | 0.898 | 0.971 | 0.620 | 1.520 |
| Age | 0.446 | 0.241 | 3.418 | 1 | 0.065 | 1.563 | 0.973 | 2.508 |
| Tumor size | 0.412 | 0.330 | 1.649 | 3 | 0.648 | 1.510 | 0.790 | 2.885 |
| Differentiation | −0.278 | 0.277 | 4.439 | 2 | 0.109 | 0.758 | 0.440 | 1.304 |
| Lymph node metastasis | 0.875 | 0.314 | 7.782 | 1 | 0.005 | 2.399 | 1.297 | 4.436 |
| TMN stage | −1.460 | 0.453 | 13.111 | 3 | 0.004 | 0.232 | 0.096 | 0.564 |
Univariate analyses of PFS by Cox model were conducted to evaluate the potential prognostic factor in GBC. The candidate factors included the level of Linc00261 expression, gender, age, tumor size and differentiated degree, lymph node metastasis and TNM stage. B means regression coefficient. SE means standard error. df means degree of freedom.
Serum Linc00261 is significantly decreased in GBC and can be used as a potential diagnostic biomarker for GBC
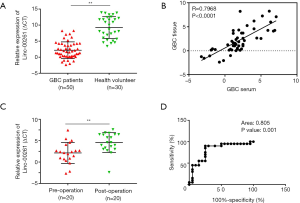
We supposed that Linc00261 expressed in serum may be affected by GBC. So, the level of Linc00261 expression in GBC patients’ or healthy volunteers’ serum had been examined by qRT-PCR. The results showed that the expression of Linc00261 was down-regulated in the serum of GBC patients (Figure 3A). Additionally, the correlation analysis was used to study the relationship between Linc00261 expression in GBC tissues and in serum of patient with GBC. As shown in Figure 3B, Linc00261 expression in GBC tissue was positively associated with its expression in GBC patient’s serum (P<0.0001). We also compared the serum Linc00261 level in GBC patients and the patients after surgery, which revealed that serum Linc00261 level was increased in the patients after operation (Figure 3C). Then diagnosis efficacy of using the serum Linc00261 level to identify the GBC was assessed. ROC curve analysis (Figure 3D) showed this diagnostic method has high specificity and sensitivity on GBC diagnosis (P=0.001, AUC 0.805).
Discussion
GBC is the most common and aggressive malignancy of the bile duct, and the worldwide incidence of the disease is increasing annually (18). Despite the recent advances in the management of GBC, the prognosis of patients with GBC remains relatively poor (19,20). The overall 5-year survival rate of GBC is less than 5%. But in early-stage disease, the 5-year survival rate reaching 75% can be achieved if stage-adjusted therapy is performed. Additionally, only a third of GBCs are preoperatively recognized. GBC is diagnosed by pathologists after routine cholecystectomy for a benign disease, which was termed ‘‘incidental or occult gallbladder carcinoma (IGBC)” (7). Therefore, it is significant that to find novel genes associated with GBC development and progression and to make them as new biomarkers for early diagnosis.
Previous studies have shown that Linc00261 has lower expression in various kinds of cancer cell such as choriocarcinoma, gastric cancer, colon cancer and esophageal cancer cells (21,22) compared with normal cells. Linc00261 has been recognized as a tumor-suppressor gene. There was report that Linc00261 can restrain β-catenin from cytoplasm into nuclei to down-regulate nuclear β-catenin, which could promote β-catenin degradation and inhibit activation of WNT pathway in human colon cancer cells (23). In gastric cancer cell lines, Linc00261 could decrease the stability of Slug proteins and suppressing epithelial-mesenchymal transition (EMT) (21). Moreover, during lung cancer tumorigenesis and progression, Linc00261 can suppress EMT correlated with its neighboring gene forkhead box A2 (FOXA2) (24). In our research, we found out that the expression of Linc00261 was decreased in GBC tissues compared with para-carcinoma tissues just like reported researches mentioned above. And the expression level of Linc00261 negatively correlated with advanced tumor status, clinical stage and poor prognostic outcome.
Many researches also identify Linc00261 as a prognostic biomarker of many malignancies (17,21,24,25). In hepatocellular carcinoma (HCC), lower expression of Linc00261 was considered as an independent risk factor affecting postoperative recurrence-free survival time of the patients (16,26). And Linc00261 with other three lncRNAs (TRELM3P, GBP1P1, and CDKN2B-AS1) were identified as significantly correlated with overall survival (OS) of HCC patients (25). Cox model analysis result revealed that Linc00261 expression was also an independent prognostic factor for survival of GBC patients in this report.
Moreover, we proposed using serum Linc00261 expression level to reflect the status of GBC. The results showed that serum Linc00261 is significantly decreased in patients with GBC. Serum Linc00261 level was also increased in the patients after surgery. Linc00261 expression in patient’s serum was positively associated with its expression in GBC tissues.
Conclusions
In conclusion, this research evidenced that Linc00261 was downregulated in GBC and its expression level was associated with OS and PFS rate in GBC, which proved that Linc00261 can be an independent potential biomarker for the prognosis of GBC. Linc00261 in tissues or serum might be used as a potential marker for the prognosis of GBC, which may contribute to early diagnosis and treatment of GBC.
Acknowledgments
Funding: This study is funded by
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1091
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1091). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the First People’s Hospital of Yunnan Province (approval number: KY-E-2017-1-20) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rawla P, Sunkara T, Thandra KC, et al. Epidemiology of gallbladder cancer. Clin Exp Hepatol 2019;5:93-102. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363-85. [Crossref] [PubMed]
- Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg 1994;219:275-80. [Crossref] [PubMed]
- Graur F, Mois E, Margarit S, et al. Gallbladder carcinoma. Surgical management of gallblad-der carcinoma. An analysis of 37 cases. Ann Ital Chir 2018;89:501-6. [PubMed]
- Goetze TO. Gallbladder carcinoma: Prognostic factors and therapeutic options. World J Gastroenterol 2015;21:12211-7. [Crossref] [PubMed]
- Hu ZH, Li ZW, Shen L, et al. Surgical therapy and prognosis of sarcomatoid carcinoma of the gallbladder. Hepatobiliary Pancreat Dis Int 2010;9:175-9. [PubMed]
- Trautman J, Wood BE, Craig SJ. A rare case report of gallbladder carcinosarcoma. J Surg Case Rep 2018;2018:rjy167. [Crossref] [PubMed]
- Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature 2012;482:339-46. [Crossref] [PubMed]
- He Y, Meng XM, Huang C, et al. Long noncoding RNAs: Novel insights into hepatocelluar carcinoma. Cancer Lett 2014;344:20-7. [Crossref] [PubMed]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 2011;12:861-74. [Crossref] [PubMed]
- Cao WJ, Wu HL, He BS, et al. Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol 2013;19:3658-64. [Crossref] [PubMed]
- Wang Y, Xue K, Guan Y, et al. Long Noncoding RNA LINC00261 Suppresses Cell Proliferation and Invasion and Promotes Cell Apoptosis in Human Choriocarcinoma. Oncol Res 2017;25:733-42. [Crossref] [PubMed]
- Müller S, Raulefs S, Bruns P, et al. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer 2015;14:94. [Crossref] [PubMed]
- Zhang HF, Li W, Han YD. LINC00261 suppresses cell proliferation, invasion and Notch signaling pathway in hepatocellular carcinoma. Cancer Biomark 2018;21:575-82. [Crossref] [PubMed]
- Liu Y, Xiao N, Xu SF. Decreased expression of long non-coding RNA LINC00261 is a prognostic marker for patients with non-small cell lung cancer: a preliminary study. Eur Rev Med Pharmacol Sci 2017;21:5691-5. [PubMed]
- Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am 2014;94:343-60. [Crossref] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [PubMed]
- Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555-67. [Crossref] [PubMed]
- Yu Y, Li L, Zheng Z, et al. Long non-coding RNA linc00261 suppresses gastric cancer progression via promoting Slug degradation. J Cell Mol Med 2017;21:955-67. [Crossref] [PubMed]
- Lin K, Jiang H, Zhuang SS, et al. Long noncoding RNA LINC00261 induces chemosensitization to 5-fluorouracil by mediating methylation-dependent repression of DPYD in human esophageal cancer. FASEB J 2019;33:1972-88. [Crossref] [PubMed]
- Wang ZK, Yang L, Wu LL, et al. Long non-coding RNA LINC00261 sensitizes human colon cancer cells to cisplatin therapy. Braz J Med Biol Res 2017;51:e6793. [Crossref] [PubMed]
- Dhamija S, Becker AC, Sharma Y, et al. LINC00261 and the Adjacent Gene FOXA2 Are Epithelial Markers and Are Suppressed during Lung Cancer Tumorigenesis and Progression. Noncoding RNA 2018;5:2. [Crossref] [PubMed]
- Sui J, Miao Y, Han J, et al. Systematic analyses of a novel lncRNA-associated signature as the prognostic biomarker for Hepatocellular Carcinoma. Cancer Med 2018;7:3240-56. [Crossref] [PubMed]
- Chen Z, Xiang L, Huang Y, et al. Nan Fang Yi Ke Da Xue Xue Bao 2018;38:1179-86. [Expression of long noncoding RNA linc00261 in hepatocellular carcinoma and its association with postoperative outcomes]. [PubMed]


