Distal pancreatectomy with en bloc celiac axis resection does not improve the R0 rate or median survival time of patients with locally advanced pancreatic cancer: a systematic review and meta-analysis
Introduction
Pancreatic tumors affecting the body or tail of the pancreas are most often identified when they are at an advanced stage and are no longer eligible for resection owing to celiac axis (CA) or common hepatic artery origin involvement (1). In patients with locally advanced pancreatic cancer, chemotherapy and radiotherapy are generally the only viable treatment options, but these approaches are associated with poor efficacy. Distal pancreatectomy with en bloc celiac axis resection (DP-CAR) is an approach that endeavors to achieve negative CA, nerve plexus, and retroperitoneal tissue margins in such patients (2), thus potentially enabling complete tumor resection. While DP-CAR may improve tumor resection rates, it is also associated with very high rates of postoperative morbidity, making the value of this procedure unclear. Indeed, short-term studies comparing DP-CAR to DP have yielded inconsistent outcomes, and the quality of life and postoperative survival of patients following DP-CAR. Some studies have found DP-CAR to offer no survival benefits relative to DP (3,4), while other studies have found that this approach can improve the duration of disease-free survival (DFS) in some patients (5). Hirano et al. (2) found DP-CAR to be associated with a 5-year overall survival (OS) rate of 42%, which was significantly longer than that associated with DP. Owing to its complexity and high morbidity and mortality rates, however, DP-CAR is not commonly performed, making it challenging to conduct large sample size studies on this procedure. Most published studies of DP-CAR to date contain relatively few cases. As such, the present study sought to comprehensively compare the relative safety and feasibility of DP-CAR and DP based upon previously published data.
We present the following article in accordance with the PRISMA Reporting Checklist (available at http://dx.doi.org/10.21037/tcr-19-2421).
Methods
Literature search
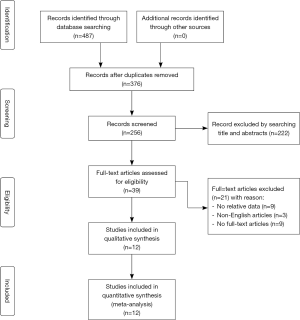
The present meta-analysis was performed in a manner consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement (6), and was registered with the PROSPERO database (number CRD42019129612). Two investigators independently searched the PubMed, EMBASE, Cochrane Library, and Web of Science databases for articles published as of June 30, 2019. Additional articles of potential relevance were identified by reviewing the references of included studies and by using the “related article” function in these databases. Only English articles were retrieved. A flow chart overview of the literature search strategy is shown in Figure 1. Studies were identified with the following search terms:
- #1 (modified appleby) OR (appleby procedure) OR (appleby operation) OR (distal pancreatectomy with en bloc celiac axis resection) OR (celiac axis resection) OR (celiac resection) OR (vascular resection) OR (vessel resection) OR (dp-car) OR (distal pancreatectomy with celiac axis resection);
- #2 (pancreatic carcinoma) OR (pancreatic cancer) OR (pancreatic neoplasm) OR (pancreatic body and tail carcinoma) OR (adenocarcinoma of the body and tail of the pancreas) OR (locally advanced pancreatic cancer) OR (pancreatic body and tail cancer) OR (pancreatic body cancer) OR (body-tail pancreatic cancer);
- #3 (pancreatectomy) OR (distal pancreatectomy);
- #1 AND #2 AND #3.
Duplicate articles were eliminated, after which the remaining studies were assessed for potential relevance through successive title, abstract, and full-text review.
Inclusion criteria
Studies relevant for inclusion in the present study were those that were: (I) studies of patients with locally advanced pancreatic cancer involving the CA, common hepatic artery origin, or root of the splenic artery; (II) studies reporting at least one clinical outcome of interest in the form of means and standard deviations for continuous variables, or reporting sufficient data to permit the calculation of these values; (III) studies of DP-CAR and DP interventions; (IV) studies that were randomized controlled trials (RCTs), cohort studies, or case-control studies. When multiple studies from the same institution or authors were identified, only the most recent or high-quality studies were included in the final meta-analysis.
Studies were excluded from this meta-analysis if they were: (I) abstracts, reviews, letters, expert opinions, case reports, editorials, or studies lacking a control group; (II) studies with fewer than 6 DP-CAR cases; or (III) not published in English, duplicates, or non-human studies.
Data extraction
Two investigators (Q Feng, L Li) independently extracted data from all included studies. These investigators first reviewed the titles and abstracts of all identified articles, after which potentially relevant studies were subjected to full-text review to confirm eligibility. Discussion with a third investigator (H Zhai) was used to resolve inconsistencies. When data were missing, efforts were made to contact the original study authors to obtain these data. Where necessary, data were estimated based upon available summary statistics or figures. Data were extracted as intention-to-treat analyses. When unavailable, per-protocol assessments were utilized. In cases where outcomes were assessed across a range of time points, outcomes measured with the longest duration were used to remove duplicate data or arbitrary variability. Evidence quality for cohort studies was assessed based upon Newcastle-Ottawa Scale (NOS), with scores of 7 or higher being indicative of a high-quality study.
Risk of bias assessment
Two investigators (Q Feng and L Li) independently evaluated the quality and risk of bias associated with each included study using the Cochrane Handbook for Systematic Reviews of Interventions risk of bias tool (7). Briefly, such risk was assessed based upon random sequence generation, allocation concealment, participant blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other bias. Discussion with a third investigator (H Zhai) was used to resolve any discrepancies.
Statistical analysis
The Cochrane Collaboration Review Manager program (v.5.3) was used to analyze all data. Odds ratios (OR) and 95% confidence intervals (CIs) were used to assess dichotomous variables, whereas mean difference (MD) and 95% CIs were used to assess continuous variables using fixed-effects or random-effects models as appropriate. P<0.05 was the significance threshol. I2 and P values were used to assess heterogeneity among included studies, with I2>50% and P<0.05 being indicative of significant heterogeneity. Funnel plots were used to visually gauge the risk of publication bias.
Results
Study identification
Our initial search strategy led to the identification of 814 potentially relevant studies after the exclusion of duplicates. Of these articles, 39 were subjected to full-text review after abstract and title reviews. Of these 39 articles, 27 were excluded, leaving 12 articles for inclusion in our final meta-analysis (7-18). The overview of this study selection process is shown in Figure 1. Of the included studies, 7 were conducted in Japan (7,8,10-12,17,18), 1 in the United States (14), 1 in France (15), 1 in Korea (13), 1 in the Netherlands (16), and 1 in China (9) (Table 1). Some articles from the same authors were included in the final analysis, as they focused on different outcomes (11,18).
Table 1
| Author (year) | Country | Study design | Inclusion period | No. of patients | Sex ratio (M/F) | Age (year) | MST (month) |
|---|---|---|---|---|---|---|---|
| DP; DP-CAR | DP; DP-CAR | DP; DP-CAR | DP; DP-CAR | ||||
| Toshihiko (in 1997) | Japan | RS | 1975 to 1994 | 19; 6 | n.r.; 4/2 | 59.5; 61.2 | n.r.; n.r. |
| Hishinuma (in 2007) | Japan | RS | 1987 to 2003 | 18; 7 | n.r.; 4/3 | n.r.; 63.8 | 25; 19 |
| Wu (in 2010) | China | RS | 2003 to 2008 | 34; 9 | 18/16; 4/5 | 62.1; 55.6 | 15; 14 |
| Takahashi (in 2011) | Japan | RS | 1993 to 2010 | 27; 16 | 17/10; 8/8 | 70; 65 | 30.9; 9.7 |
| Yamamoto (in 2012) | Japan | RS | 1991 to 2009 | 58; 13 | 39/19; 10/3 | 66; 64 | 21.1; 20.8 |
| Okada (in 2013) | Japan | RS | 2005 to 2010 | 36; 16 | 23/13; 11/5 | 68; 63 | 32; 25 |
| Hyemin (in 2015) | Korea | RS | 2000 to 2014 | 31; 7 | 16/15; 3/4 | 67.5; 58.0 | 25; 15 |
| Beane (in 2015) | USA | RS | 2011 to 2012 | 172; 20 | 57/115; 6/14 | 66; 64 | n.r.; n.r. |
| Perinel (in 2016) | France | RS | 2008 to 2014 | 66; 14 | 36/30; 9/5 | 67; 65 | 16; 17 |
| Sugiura (in 2016) | Japan | RS | 2002 to 2014 | 76; 16 | 44/32; 10/6 | 71; 70 | 43.1; 17.5 |
| Peters (in 2016) | Netherlands | RS | 2004 to 2016 | 51; 17 | 29/22; 9/8 | 67; 64.5 | 19; 20 |
| Yamamoto (in 2017) | Japan | RS | 2001 to 2012 | 323; 72 | 194/129; 40/32 | 69; 66 | 28.6; 17.5 |
DP, distal pancreatectomy; DP-CAR, distal pancreatectomy with celiac axis resection; M/F, male/female; MST, median survival time; RS, retrospective study; n.r., not report.
Intraoperative outcomes
Operative duration
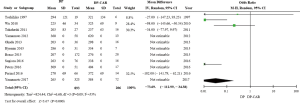
Operative duration was detailed in 11 of the included studies (8-18), with mean total operative durations of 362 and 258 minutes in the DP-CAR and DP groups, respectively. These times differed significant between groups (MD =−73.69, 95% CI: −112.99 to −34.38, P for effect =0.0002, P for heterogeneity =0.09, I2=55%) (Figure 2).
Intraoperative blood loss
In total, 10 studies reported intraoperative blood loss data for 907 patients (8-13,15-17), revealing mean blood loss values ranging from 428–1,777 mL. As significant heterogeneity was observed among included studies, a random-effects model was used for data analysis, revealing DP-CAR to be associated with higher intraoperative blood loss relative to DP (MD 186.70 mL, 95% CI: −0.33 to 373.73 mL, P=0.05).
Blood transfusion
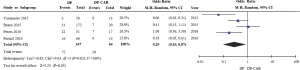
Blood transfusion rates were only reported in four of the included studies (11,14-16), revealing higher rates of blood transfusion in the DP-CAR group relative to the DP group (OR 0.29, 95% CI: 0.10 to 0.87; P for effect =0.03, P for heterogeneity =0.02, I2=69%) (Figure 3).
Portal vein (PV) resection
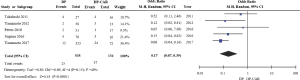
Rates of PV resection were reported in just 5 of the included studies (10,11,16-18), revealing DP-CAR to be associated with significantly higher PV resection rates relative to DP (OR 6.00, 95% CI: 2.59 to 13.91; P for effect <0.0001, P for heterogeneity =0.15, I2=40%) (Figure 4).
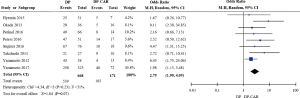
R0 resection rate
Eight of the included studies reported on rates of R0 resection for 839 patients (10-13,15-18), demonstrating that the R0 resection rate was significantly lower for DP-CAR relative to DP (OR 2.79, 95% CI: 1.90 to 4.09; P for effect <0.00001, P for heterogeneity =0.43, I2=0%) (Figure 5).
Tumor size
Six of the included studies reported tumor size data (9,10,13,15,16,18), revealing no significant differences between the DP-CAR and DP groups with respect to such size (MD −5.04, 95% CI: −15.18 to 5.10, P for effect =0.33, P for heterogeneity =0.02, I2=76%).
Postoperative outcomes
Postoperative morbidity
Nine of the included studies reported on patient morbidity rates (7,10-12,14-18), revealing no significant differences in such morbidity between the DP-CAR and DP groups (OR 0.69, 95% CI: 0.41 to 1.14; P for effect =0.15, P for heterogeneity =0.11, I2=38%).
Postoperative mortality
Eight of the included studies reported on patient mortality rates (7,8,10-12,14,16,18), indicating no significant differences in these rates between these two groups (OR 0.40, 95% CI: 0.15 to 1.06; P for effect =0.07, P for heterogeneity =0.23, I2=31%).
Postoperative pancreatic fistula (POPF)
POPF incidence rates were described for 340 patients in 10 of the studies included in this meta-analysis (9-18). Of these studies, 8 defined POPF as per the criteria of the International Study Group on Pancreatic Fistula. No significant differences in POPF rates were observed between these two groups (OR 1.15, 95% CI: 0.79 to 1.66; P for effect =0.36, P for heterogeneity =0.61, I2=0%), and no evidence of publication bias was observed.
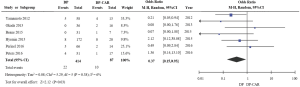
Delayed gastric emptying (DGE)
In total, six of the studies in this meta-analysis reported on DGE (11-16), and indicated that DGE rates differed significantly between the DP-CAR and DP patient cohorts (OR 0.37, 95% CI: 0.15 to 0.93, P for effect =0.03, P for heterogeneity =0.38, I2=6%) (Figure 6).
Discussion
Advanced pancreatic cancer is often not eligible for resection owing to CA involvement at the time of initial diagnosis. DP-CAR approaches can dramatically increase the resectability of tumors by completely resecting both the tumor and involved vessels. Hirano et al. (2) initially reported a series of DP-CAR cases associated with lower R1 resection rates and an acceptable 21-month median OS among treated patients. However, more recent studies have yielded outcomes inconsistent with this initial report, with median OS durations ranging from 9.3 to 26 months (9-11). To date, only small sporadic retrospective analyses of DP-CAR have been published, and these studies lack clear comparisons of postoperative outcomes between DP-CAR and standard DP patient cohorts. As such, it remains uncertain as to whether DP-CAR is an effective approach to improving survival outcomes in individuals suffering from locally advanced pancreatic body/tail cancer.
In an effort to overcome this uncertainty, we conducted a meta-analysis comparing the DP-CAR and DP approaches, revealing that DP-CAR is a complex procedure that is associated with high postoperative morbidity but also with acceptable postoperative survival and quality of life in treated patients. Rates of microscopically curative (R0) resection were found to be significantly decreased in the DP-CAR group relative to the DP group (55.55% vs. 80.68%, P<0.0001). DP-CAR was also associated with prolonged operative duration and greater intraoperative blood loss and the need for transfusion relative to DP. Rates of postoperative mortality and morbidity were high in DP-CAR-treated patients, but did not differ significantly from those in patients that underwent DP. The most common complications of DP-CAR were DGE and POPF, with a postoperative POPF incidence rate of 31.31% (95% CI: 23.69% to 40.12%) following DP-CAR that was comparable to that following DP. Rates of DGE were significantly higher among patients that underwent DP-CAR relative to patients that underwent DP. While most studies have found DP-CAR to be associated with significant improvements in patient quality of life, the association between this treatment and patient OS remains uncertain. Rates of 5-year OS varied from 0–42% in published studies following DP-CAR (2,19), with a median OS of 9–26 months in patients with advanced pancreatic cancer. When institutional data regarding patient outcomes were reviewed, an apparent trend towards increased OS was evident when specifically assessing patients with borderline resectable or locally advanced pancreatic body or tail tumors that underwent DP-CAR relative to patients not given the opportunity to undergo resection. Just 10 of the 12 studies included in this analysis reported on survival outcomes, with median survival durations of 14–30.9 months. Overall, our analysis found that DP-CAR exhibited a median 17.7 month survival.
DP-CAR was linked with reduced OS relative to DP. This finding was not surprising, given that patients that underwent DP-CAR generally had more advanced disease. No RCTs analyzing DP-CAR have been published to date, and as such all data included herein was derived from retrospective studies with limited clinical evidence and small sample sizes. Many years or decades may be required to obtain a series of >10 DP-CAR cases in a clinical setting, and the mean interval for studies in the present meta-analysis ranged from 4–19 years. As such, these study outcomes may be affected by variations in treatment protocols and perioperative management over these prolonged intervals. Some evidence of heterogeneity and possible publication bias was detected when analyzing certain outcomes.
In those that have advanced or borderline resectable pancreatic cancer, preoperative neoadjuvant chemoradiotherapy has been used in an effort to improve patient outcomes. Baumgartner et al. (5). found that DP-CAR following neoadjuvant chemoradiotherapy in those that have locally advanced pancreatic cancer was associated with higher local control rates, with a 91% R0 resection rate and a 26-month median OS. Cesaretti et al. (20) found that 5/7 cases of borderline resectable/locally advanced pancreatic cancer were able to undergo DP-CR following neoadjuvant chemotherapy, with a median OS of 24 months. This approach offers multiple advantages, including preoperative disease downstaging that can aid in achieving R0 resection, early micrometastasis treatment, and the identification of patients most likely to benefit from DP-CAR treatment.
Conclusions
In summary, in the present meta-analysis we determined that DP-CAR represents a reasonable approach to treating those that have locally advanced pancreatic body/tail cancer, as this approach is associated with a median OS of 17.7 months. This approach is also associated with an acceptable resectability rate and benefits to postoperative patient survival. Even so, this is a high-risk and complex procedure that can only be conducted by skilled surgeons. In addition, this study has a number of limitations, and as such, the relative value of DP-CAR as a treatment approach remains to be established, and our results must be interpreted with caution. Future large-scale prospective comparative studies and randomized clinical trials will be essential in order to firmly establish the safety and long-term efficacy of DP-CAR.
Acknowledgments
Funding: This work was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the PRISMA Reporting Checklist. Available at http://dx.doi.org/10.21037/tcr-19-2421
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2421). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Langlois M, Lemay F, Beaudoin A. A250 unresequable and metastatic pancreatic adenocarcinoma in the elderly: a 10-year single-center experience. J Can Assoc Gastroenterol 2018;1:435-6. [Crossref]
- Hirano S, Kondo S, Hara T, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg 2007;246:46-51. [Crossref] [PubMed]
- Yamaguchi K, Nakano K, Kobayashi K, et al. Appleby operation for pancreatic body-tail carcinoma: report of three cases. Surg Today 2003;33:873-8. [Crossref] [PubMed]
- Mizutani S, Shioya T, Maejima K, et al. Two successful curative operations using stomach-preserving distal pancreatectomy with celiac axis resection for the treatment of locally advanced pancreatic body cancer. J Hepatobiliary Pancreat Surg 2009;16:229-33. [Crossref] [PubMed]
- Baumgartner JM, Krasinskas A, Daouadi M, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic adenocarcinoma following neoadjuvant therapy. J Gastrointest Surg 2012;16:1152-9. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30. [PubMed]
- Hishinuma S, Ogata Y, Tomikawa M, et al. Stomach-preserving distal pancreatectomy with combined resection of the celiac artery: radical procedure for locally advanced cancer of the pancreatic body. J Gastrointest Surg 2007;11:743-9. [Crossref] [PubMed]
- Mayumi T, Nimura Y, Kamiya J, et al. Distal pancreatectomy with en bloc resection of the celiac artery for carcinoma of the body and tail of the pancreas. Int J Pancreatol 1997;22:15-21. [Crossref] [PubMed]
- Wu X, Tao R, Lei R, et al. Distal pancreatectomy combined with celiac axis resection in treatment of carcinoma of the body/tail of the pancreas: a single-center experience. Ann Surg Oncol 2010;17:1359-66. [Crossref] [PubMed]
- Takahashi Y, Kaneoka Y, Maeda A, et al. Distal pancreatectomy with celiac axis resection for carcinoma of the body and tail of the pancreas. World J Surg 2011;35:2535-42. [Crossref] [PubMed]
- Yamamoto Y, Sakamoto Y, Ban D, et al. Is celiac axis resection justified for T4 pancreatic body cancer? Surgery 2012;151:61-9. [Crossref] [PubMed]
- Okada K, Kawai M, Tani M, et al. Surgical strategy for patients with pancreatic body/tail carcinoma: who should undergo distal pancreatectomy with en-bloc celiac axis resection? Surgery 2013;153:365-72. [Crossref] [PubMed]
- Ham H, Kim SG, Kwon HJ, et al. Distal pancreatectomy with celiac axis resection for pancreatic body and tail cancer invading celiac axis. Ann Surg Treat Res 2015;89:167-75. [Crossref] [PubMed]
- Beane JD, House MG, Pitt SC, et al. Distal pancreatectomy with celiac axis resection: what are the added risks? HPB (Oxford) 2015;17:777-84. [Crossref] [PubMed]
- Perinel J, Nappo G, El Bechwaty M, et al. Locally advanced pancreatic duct adenocarcinoma: pancreatectomy with planned arterial resection based on axial arterial encasement. Langenbecks Arch Surg 2016;401:1131-42. [Crossref] [PubMed]
- Peters NA, Javed AA, Cameron JL, et al. Modified Appleby Procedure for Pancreatic Adenocarcinoma: Does Improved Neoadjuvant Therapy Warrant Such an Aggressive Approach? Ann Surg Oncol 2016;23:3757-64. [Crossref] [PubMed]
- Sugiura T, Okamura Y, Ito T, et al. Surgical Indications of Distal Pancreatectomy with Celiac Axis Resection for Pancreatic Body/Tail Cancer. World J Surg 2017;41:258-66. [Crossref] [PubMed]
- Yamamoto T, Satoi S, Kawai M, et al. Is distal pancreatectomy with en-bloc celiac axis resection effective for patients with locally advanced pancreatic ductal adenocarcinoma? -Multicenter surgical group study. Pancreatology 2018;18:106-13. [Crossref] [PubMed]
- Hirano S, Kondo S, Tanaka E, et al. Postoperative bowel function and nutritional status following distal pancreatectomy with en-bloc celiac axis resection. Dig Surg 2010;27:212-6. [Crossref] [PubMed]
- Cesaretti M, Abdel-Rehim M, Barbier L, et al. Modified Appleby procedure for borderline resectable/locally advanced distal pancreatic adenocarcinoma: A major procedure for selected patients. J Visc Surg 2016;153:173-81. [Crossref] [PubMed]