
Acquired heparin-like anticoagulation process in a patient with multiple myeloma: a case report and literature review
Introduction
Diverse hemostatic abnormalities have been reported in patients with multiple myeloma (MM) which predispose to bleeding and also thrombosis. Several mechanisms have been proposed to explain this phenomenon, such as inhibitors of coagulation factors, direct inhibition of fibrin monomer polymerization or presence of M protein binding to von Willebrand factor (1-4). Another uncommon cause of bleeding in patients with MM is the presence of heparin-like anticoagulant (5-11), which is also observed in solid tumors (12,13), chronic renal disease and other hematological malignancies such as chronic lymphocytic leukemia and T cell lymphoma (14,15). In the present report, the specific case of a middle-aged male diagnosis with a rare type of MM which is IgD lambda light chain and lambda light chain, presenting obvious abnormal coagulation profiles which showed the presence of heparin-like anticoagulant, was treated with chemotherapy and then the coagulation functions were corrected. We present the following case in accordance with the CARE reporting checklist (16) (available at http://dx.doi.org/10.21037/tcr-20-1968).
Case presentation
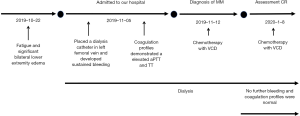
A 48-year-old man was admitted to the first affiliated hospital of Nanjing Medical University, Jiangsu Province Hospital on November 2019 because of fatigue and significant bilateral lower extremity edema on physical examination. Throughout the duration of symptoms, the patient did not have hematuria, epistaxis, hematochezia, oral ulcers, skin lesions or arthralgia. The patient had no medical history, bleeding history or family history of bleeding. The timeline of this patient is shown in Figure 1. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Jiangsu Province Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Laboratory studies revealed an elevated white blood count of 24.44×109/L, elevated platelet count of 339×109/L, decreased hemoglobin of 63 g/L, while percentage of reticulocytes was 6.60%. Total serum protein was 61.3 g/L (65–85 g/L) with 28.7 g/L (40–55 g/L) albumin and 32.6 g/L (20–40 g/L) globulin, calcium of 2.12 mmol/L (2.2–2.65 mmol/L), and creatinine of 936.2 mmol/L (44–133 mmol/L). The level of IgG, IgA, IgM and IgD were 1.61 g/L, <0.24 g/L, <0.175 g/L and 4.42 g/L respectively. The serum free light-chain (FLC) of kappa was 17.6mg/L and lambda was 2,400 mg/L, the ratio of kappa/lambda was 0.007. Serum protein electrophoresis (SPEP) showed a M protein of 9.9% and serum immunofixation electrophoresis (SIFE) revealed two oligoclonal bands, IgD lambda light chain and lambda light chain. The 24-hour urine for total protein was 11.1g and the urine immunofixation electrophoresis (UIFE) showed a monoclonal of lambda light chain. Serum beta-2 microglobulin was 27.1 mg/L and LDH was 262 U/L (140–271 U/L). The bone marrow smear showed primary plasma cells account for 30.4% and flow cytometry revealed an abnormal plasma cell population, expressing CD38, CD138, CD56 and lambda light chains. Hematopathology revealed extensive bone marrow involvement (40%) by plasma cells. Chromosome was normal and the Fluorescence in situ hybridization (FISH) of MM was negative. So patient was diagnosed with multiple myeloma (IgD lambda + lambda, ISS III, R-ISS II).
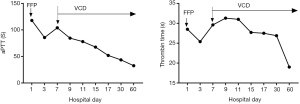
Notably, because of acute renal insufficiency, a dialysis catheter was placed in the left femoral vein. The patient developed sustained bleeding at the site of puncture. The coagulation profiles demonstrated that mildly elevated prothrombin time (PT) at 14 s (normal range 8.0–14.0 s) and a significantly elevated activated partial thromboplastin time (aPTT) at 117.9 s (25.0–31.3 s) and thrombin time (TT) at 28.5 s (15–21 s), while the reptilase time (RT) was normal. Addition of an equal volume of normal plasma did not correct the aPTT completely. The level of coagulation factors was listed in Table 1. The test of lupus anticoagulant, antiphospholipid syndrome, antinuclear antibodies and antineutrophil cytoplasmic antibodies were normal. The prolonged TT (31.9 s) was corrected to 16.2 s with in vitro addition of 100 µg (5 µg/µL) protamine sulfate, which indicated the presence of a heparin-like anticoagulant. No heparin or other anti-coagulants were administered at any time. The infusion of fresh-frozen plasma had no significant effect on the coagulation studies or the bleeding time. After treatment of 2 cycles of bortezomib (1.3 mg/m2, day 1, 4, 8, 11) combined with cyclophosphamide (CTX) (300 mg/m2, day 1, 8, 15) and dexamethasone (Dex) (20 mg, day 1–2, 4–5, 8–9, 11–12) therapy, the SPEP, SIFE and UIFE were negative and FLC was normal, meanwhile the patient did not show any other signs of bleeding and the aPTT and TT were back to normal (Figure 2). The patient showed no adverse and unanticipated events as of the last follow- up on January 2020 (Figure 1).
Table 1
| Coagulation factors | Before treatment (Activity %) |
8 times dilution (Activity %) |
After 2 cycles of treatment (Activity %) | Normal range |
|---|---|---|---|---|
| Factor II | 160.6 | – | 76.2 | (70–120) |
| Factor V | 139.5 | – | 112.4 | (70–120) |
| Factor VII | 173.2 | – | 94.7 | (70–120) |
| Factor VIII | 60.7 | 202.6 | 153.8 | (70–150) |
| Factor IX | 49.9 | 205.1 | 78.6 | (70–120) |
| Factor X | 152 | – | 78.5 | (70–120) |
| Factor XI | 24.4 | 64.9 | 81.9 | (70–120) |
| Factor XII | 13.9 | 36.8 | 31.5 | (70–150) |
| Protein C | 122.6 | – | ||
| vWF | 404.9 | – | (49.5–187) |
vWF, von Willebrand factor.
Discussion
Our patient was diagnosed with MM and presented with sustained bleeding at the puncture site. The coagulation profile is remarkable for prolonged aPTT and TT, while mixing test could not correct the aPTT. This suggested the presence of inhibitor of coagulation factors or lupus anticoagulant. The test of coagulation factors showed low activity of factor VIII, factor IX, factor XI and factor XII. However, the fact that the activities of these coagulant factors elevated after 8 times dilution of the plasma demonstrated that no specific inhibitors of these coagulation factors existed. Meantime the lupus anticoagulant was negative. The TT was corrected by the addition of protamine sulfate, which suggested a heparin-like anticoagulant.
Several MM patients were reported to have heparin-like anticoagulants with mild to severe hemorrhage (Table 2). The source of acquired heparin-like anticoagulants is poorly understood. Some authors believe that these substances are derived from myeloma cells. Lamia Torjemane et al. considered that soluble CD138 may play a role in generating heparin-like anticoagulants (5), but there is no clear evidence to support this view. Martínez-Martínez et al. found that IgG could act as heparin binding the heparin binding domain of antithrombin which may contribute to the risk of bleeding of MM patients (9). We started chemotherapy (bortezomib combined with cyclophosphamide and dexamethasone) on the patient after he was diagnosis with MM. The normalization of TT with the reduction of the tumor burden may support this hypothesis. Of note, in the case reported by Martínez-Martínez et al., the patient had an IgG-gamma myeloma, while our patient had an IgD lambda + lambda, which is a rare type of MM.
Table 2
| No. | Age | Gender | Diagnosis | PT (s) | aPTT (s) | TT (s) | RT (s) | Fbg (g/L) | Subtypes of myeloma | At the time of diagnosis | Treatment | Prognosis | Article |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | Female | MM | 21 | 48 | >600 | 21 | 138 | IgA kappa | Yes | Cryoprecipitate + plasmapheresis + protamine | Dead of sepsis | Tefferi 1990 (6) |
| 2 | 46 | Male | MGUS | 18 | 46 | 105 | 18 | 626 | IgG kappa | Yes | Protamine +corticosteroids + plasmapheresis | Dead of sepsis and bleeding | |
| 3 | 47 | Male | MM | 25 | 44 | >600 | 17 | 432 | IgG lambda | Yes | L-phenylalanine mustard + prednisone | Dead of renal failure and coronary artery disease | |
| 4 | 78 | Female | MM | 28 | 48 | >600 | 20 | 883 | Kappa | Yes | Chemotherapy + protamine | Dead of sepsis and bleeding | |
| 5 | – | – | MM | 15 | 35.4 | 122 | 22 | – | IgG + IgA | Yes | Protamine | Unknown | Bayer-Garner 2001 (11) |
| 6 | 55 | Male | MM | 14 | 63 | 65 | 22 | 267 | IgG lambda | No | Chemotherapy + ASCT | Unknown | Torjemane 2007 (5) |
| 7 | 73 | Female | MM | – | – | – | – | – | IgG | Yes | Recombinant FVIIa + chemotherapy | PR and no further bleeding events | Martínez-Martínez 2016 (9) |
| 8 | 62 | Female | MM | 12 | 44.3 | 32.3 | 22 | 228 | IgG kappa | No | Protamine | No further bleeding events | Willner 2018 (8) |
PT, prothrombin time; aPTT, activated partial thromboplastin time; TT, thrombin time; RT, reptilase time; Fbg, fibrinogen; MM, multiple myeloma; MGUS, monoclonal gammopathy of undetermined significance.
It is worth noting that not all patients had abnormal coagulation at the time of diagnosis. Some patients acquired heparin-like anticoagulants after the treatment, while their MM was in remission. And some primary amyloid patients without detectable serum M-proteins also had prolonged TT, suggesting that the heparin-like anticoagulants may not derived from M-proteins (17,18).
There is a lack of literature on treatment of MM patients with acquired heparin-like anticoagulants. According to existent literature, the choice of treatment is mainly based on the degree of bleeding. Patients with severe, life-threatening bleeding may benefit from infusing with protamine sulfate which can neutralize the heparin-like anticoagulants (5,6), while no guidelines on dosing is established yet. Plasmapheresis may also be effective in some cases (19). For our patient who had non-life-threatening bleeding, chemotherapy for MM did correct the prolonged TT and stopped the bleeding.
No association has been drew between the existence of heparin-like anticoagulants and poorer prognosis among MM patients. This also supports that the heparin-like anticoagulants may not be exclusively secreted by myeloma cells. According to the report of Tefferi et al., their patients all died of bleeding or sepsis, rather than the primary disease.
In our case, this patient is diagnosed with MM of IgD lambda light chain and lambda light chain, which is a rare type of MM, and no heparin-like anticoagulants has been reported in patients with this type of MM. It is a pity that we did not extract M-protein from patient’s peripheral blood for further verification, so we cannot directly prove that the source of the heparin-like anticoagulant is the M protein. Chemotherapy can clear the M protein, but the effect on heparin-like anticoagulants needs further study.
In summary, heparin-like anticoagulants are uncommon in MM patients, and bleeding may fatal, early evaluation of the coagulation function of MM patients is clinically important for early intervention. In order to better understand the origin and roles of these anticoagulants, future research are warranted.
Acknowledgments
We thank all members of our group for their efforts and enthusiasm.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1968
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1968). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Jiangsu Province Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Quek JK, Wong WH, Tan CW, et al. Acquired factor V deficiency in a patient with myeloma and amyloidosis. Thromb Res 2018;164:1-3. [Crossref] [PubMed]
- Muzaffar J, Katragadda L, Haider S, et al. Rituximab and intravenous immunoglobulin (IVIG) for the management of acquired factor VIII inhibitor in multiple myeloma: case report and review of literature. Int J Hematol 2012;95:102-6. [Crossref] [PubMed]
- Shinagawa A, Kojima H, Kobayashi T, et al. Lupus anticoagulant-like activity observed in a dimeric lambda protein produced by myeloma cells. Int J Hematol 2001;73:526-31. [Crossref] [PubMed]
- Post GR, James L, Alapat D, et al. A case of acquired dysfibrinogenemia in multiple myeloma treated with therapeutic plasma exchange. Transfus Apher Sci 2013;48:35-8. [Crossref] [PubMed]
- Torjemane L, Guermazi S, Ladeb S, et al. Heparin-like anticoagulant associated with multiple myeloma and neutralized with protamine sulfate. Blood Coagul Fibrinolysis 2007;18:279-81. [Crossref] [PubMed]
- Tefferi A, Nichols WL, Bowie EJ. Circulating heparin-like anticoagulants: report of five consecutive cases and a review. Am J Med 1990;88:184-8. [Crossref] [PubMed]
- Chapman GS, George CB, Danley DL. Heparin-like anticoagulant associated with plasma cell myeloma. Am J Clin Pathol 1985;83:764-6. [Crossref] [PubMed]
- Willner CA, Chisti MM. Treatment of Bleeding Diathesis Associated with a Heparin-Like Anticoagulant in Plasma Cell Neoplasia Using Protamine. Case Rep Hematol 2018;2018:4342301. [Crossref] [PubMed]
- Martínez-Martínez I, González-Porras JR, Cebeira MJ, et al. Identification of a new potential mechanism responsible for severe bleeding in myeloma: immunoglobulins bind the heparin binding domain of antithrombin activating this endogenous anticoagulant. Haematologica 2016;101:e423-e426. [Crossref] [PubMed]
- Palmer RN, Rick ME, Rick PD, et al. Circulating heparan sulfate anticoagulant in a patient with a fatal bleeding disorder. N Engl J Med 1984;310:1696-9. [Crossref] [PubMed]
- Bayer-Garner IB, Sanderson RD, Dhodapkar MV, et al. Syndecan-1 (CD138) Immunoreactivity in Bone Marrow Biopsies of Multiple Myeloma: Shed Syndecan-1 Accumulates in Fibrotic Regions. Mod Pathol 2001;14:1052-8. [Crossref] [PubMed]
- Rodgers GM, Corash L. Acquired heparinlike anticoagulant in a patient with metastatic breast carcinoma. West J Med 1985;143:672. [PubMed]
- Wages DS, Staprans I, Hambleton J, et al. Structural characterization and functional effects of a circulating heparan sulfate in a patient with hepatocellular carcinoma. Am J Hematol 1998;58:285-92. [Crossref] [PubMed]
- Cetingil AI, Ulutin ON, Karaca M. Heparin-like Anticoagulant Occuring in Association with Chronic Nephritis. Br Med J 1959;2:38-9. [Crossref] [PubMed]
- Llamas P, Outeirino J, Espinoza J, et al. Report of three cases of circulating heparin-like anticoagulants. Am J Hematol 2001;67:256-8. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Gastineau DA, Gertz MA, Daniels TM, et al. Inhibitor of the thrombin time in systemic amyloidosis: a common coagulation abnormality. Blood 1991;77:2637-40. [Crossref] [PubMed]
- Mumford AD, O'Donnell J, Gillmore JD, et al. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol 2000;110:454-60. [Crossref] [PubMed]
- Goddard IR, Stewart WK, Hodson BA, et al. Plasma exchange as a treatment for endogenous glycosaminoglycan anticoagulant induced haemorrhage in a patient with myeloma kidney. Nephron 1990;56:94-6. [Crossref] [PubMed]


