
Fluconazole is as effective as other anti-mold agents in preventing early invasive fungal disease after allogeneic stem cell transplantation: assessment of antifungal therapy in haematological disease in China
Introduction
Allogeneic stem cell transplantation (allo-HSCT) had been regarded as a potentially curative treatment for haematological malignancies (1). However, it can lead to several complications, such as infections, graft-versus-host disease (GVHD), and injuries to the liver, kidneys, and lungs (2). Invasive fungal disease (IFD) is a common complication after allo-HSCT (3) that is associated with an increased risk of mortality and poor transplant outcome (4,5). Clinicians therefore commonly administer antifungal prophylaxis to reduce the risk of IFD after allo-HSCT, and previous studies have verified the efficacy of these treatments (6,7). Polyenes (amphotericin B), azoles (fluconazole, voriconazole, itraconazole, posaconazole), and echinocandins (caspofungin, micafungin) are the three main classes of antifungals used for treatment of IFD (8). Because of the limitations in current antifungal therapies, including toxicities, drug interactions, restricted routes of administration, and drug-resistance, there is a need for novel IFD treatments (9). However, several novel drugs are under development or in ongoing clinical studies, such as Rezafungin (CD101), Ibrexafungerp (SCY-078), Fosmanogepix (APX001), VT-1129, VT-1161 and VT-1598, Encochleated Amphotericin B (MAT2203), Aureobasidin A (AbA), and others (9).
The drugs used for antifungal prophylaxis include the anti-yeast agent fluconazole and anti-mold agents, such as voriconazole, itraconazole, posaconazole, amphotericin, and echinocandins. Fluconazole lacks activity against Aspergillus. There has therefore been an increased use of mold-active drugs during recent years because the dominant infectious fungi have shifted from Candida to Aspergillus (3,10-12). There is currently some doubt about the efficacy of fluconazole for prevention of IFD after allo-HSCT. In fact, previous trials comparing mold-active drugs with fluconazole have yielded inconsistent results, with most studies showing that mold-active drugs provided no greater reduction in proven or probable IFD or improvement in overall survival (13-17). Furthermore, the balance of benefits and toxicities of antifungal drugs must be considered when selecting a treatment. There are major concerns associated with the use of second-generation triazoles, namely, toxicity, drug-drug interactions, and high cost. In contrast, fluconazole is well tolerated, has activity against several yeast species, and is suitable for post-transplant patients because of its favorable tolerability and minimal drug-drug interactions.
It is noteworthy that no single anti-fungal agent is suitable in all situations. The risk of IFD and the causative pathogens vary among different allo-SCT populations for individual patients at different times after transplantation (18). Thus, a single individual typically experiences changing risk of IFD at different times after transplantation (19,20). After allo-HSCT, patients can be divided into three phases, based on the risk of opportunistic infection: an early phase (usually within 40 days), characterized by neutropenia and mucositis; a late phase (40 to 100 days), characterized by acute graft versus-host disease (GVHD); and a very late phase (after 100 days), characterized by chronic GVHD. Thus, there are marked epidemiological differences in IFDs that occur during different phases. However, the effect of different prophylactic drugs on different populations and at different times after allo-HSCT remains unclear.
Thus, we evaluated the effects of different antifungal prophylactic agents on IFD when administered at different times after transplantation. Herein, we report the findings of the China Assessment of Antifungal Therapy in Hematological Disease (CAESAR) study, the first large-scale observational study of the epidemiology, risk factors, management, and prognosis of IFD among adults and children undergoing HSCT in China.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2887).
Methods
Patients
The CAESAR study was a multicenter, prospective, observational study that enrolled patients throughout China from January 1, 2011, to October 30, 2011. The overall study methods were described previously (3). The present study focused on patients who underwent HSCT at 31 HSCT centers. Patients who had no IFD history before conditioning and who received a single drug for antifungal prophylaxis were included. Only 7 patients received caspofungin or amphotericin as prophylaxis, so they were excluded from the study to reduce analysis bias. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Peking University, People’s Hospital (NO. V20100812) and informed consent was taken from all the patients.
Definitions
IFD was defined according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (21). Also, using these criteria, IFD was categorized as proven, probable, or possible (21). Patients with possible IFD were not included. The early phase was defined as within 40 days after allo-HSCT, the late phase as 40 to 100 days after allo-HSCT, and the very late phase as more than 100 days after allo-HSCT.
Statistical analysis
Statistics were primarily descriptive and data were compared using analysis of variance, the Wilcoxon rank-sum test, or the chi-squared test in SAS version 9.1, as appropriate. The incidence of IFD was calculated as cumulative incidence of proven and probable IFD. The risk factors for IFD were analyzed using univariate analysis followed by multivariate analysis with analysis of antifungal prophylaxis method (fluconazole vs. mold-active drugs), transplantation type, and other factors. Each risk factor with a P value below 0.15 in the univariate analysis was examined using multivariate analysis, in which clinical significance and interaction between variables were considered. The overall survival was determined from the time of engraftment to six months later using the Kaplan-Meier method, and subgroups were compared using the log-rank test. The risk factors for death were analyzed by univariate analysis, and each factor with a P value below 0.10 was included as a covariate in multivariate analysis using a Cox proportional hazard regression model. Factors with P values below 0.05 in the final analysis were considered statistically significant.
Results
Characteristics of the study population
A total of 1,401 patients were screened in the CEASAR study, and 1,175 of them received antifungal prophylaxis. For the 906 recipients of allo-HSCT, 667 received a single drug for antifungal prophylaxis after allogeneic stem cell transplantation were further selected. Four patients who received caspofungin and 2 patients who received amphotericin were excluded to reduce analysis bias. Eventually, 661 patients who fulfilled our criteria were enrolled, including 429 who received fluconazole, 103 who received itraconazole capsules, 71 who received voriconazole, and 58 who received micafungin (Table 1). There were 273 (41.3%) patients who received HSCT from matched sibling donors, 198 (30.0%) from haploidentical related donors, and 189 (28.6%) from unrelated donors. Data regarding donor type were missing for one patient.
Table 1
| Variable | Fluconazole (n=429) | Itraconazole (n=103) | Voriconazole (n=71) | Micafungin (n=58) | P |
|---|---|---|---|---|---|
| Age (years) | 26 (1–63) | 30 (7–58) | 28 (6–53) | 29 (2–63) | 0.0090 |
| Gender | 0.4423 | ||||
| Male | 264 (61.5%) | 64 (62.1%) | 48 (67.6%) | 31 (53.4%) | |
| Female | 165 (38.5%) | 39 (37.9%) | 23 (32.4%) | 27 (46.6%) | |
| ECOG scoreb | <0.0001 | ||||
| 0 | 111 (25.9%) | 12 (11.7%) | 27 (38.0%) | 36 (62.1%) | |
| 1 | 223 (52.0%) | 79 (76.7%) | 28 (39.4%) | 14 (24.1%) | |
| 2 | 53 (12.4%) | 11 (10.7%) | 7 (9.9%) | 2 (3.4%) | |
| 3 | 33 (7.7%) | 1 (1.0%) | 8 (11.3%) | 5 (8.6%) | |
| 4 | 9 (2.1%) | – | 1 (1.4%) | 1 (1.7%) | |
| Primary disease | 0.4481 | ||||
| Acute lymphocytic leukemia | 140 (32.6%) | 31 (30.1%) | 16 (22.5%) | 15 (25.9%) | |
| Aplastic anemia | 35 (8.2%) | 4 (3.9%) | 8 (11.3%) | 7 (12.1%) | |
| Acute myelogenous leukaemia | 146 (34.0%) | 48 (46.6%) | 32 (45.1%) | 14 (24.1%) | |
| Chronic myelogenous leukaemia | 48 (11.2%) | 10 (9.7%) | 4 (5.6%) | 10 (17.2%) | |
| Non-Hodgkin lymphoma | 12 (2.8%) | 4 (3.9%) | 3 (4.2%) | 2 (3.4%) | |
| Myelodysplastic syndrome | 29 (6.8%) | 5 (4.9%) | 5 (7.0%) | 7 (12.1%) | |
| Othersc | 19 (4.4%) | 1 (1.0%) | 3 (4.2%) | 3 (5.2%) | |
| Duration of prophylaxis, daysd | 32 (1–117) | 41 (4–115) | 35 (1–376) | 24 (4–81) | <0.0001 |
| Cytomegalovirus viremia | 0.0012 | ||||
| Yes | 131 (30.5%) | 42 (40.8%) | 12 (16.9%) | 17 (29.3%) | |
| No | 290 (67.6%) | 58 (56.3%) | 51 (71.8%) | 39 (67.2%) | |
| Untested | 8 (1.9%) | 3 (2.9%) | 8 (11.3%) | 2 (3.4%) | |
| Time to neutrophil recovery | 14 (3–44) | 14 (8–32) | 13 (9–26) | 14 (10–34) | 0.1693 |
| Time to platelet recovery | 15 (3–128) | 15 (9–72) | 14 (8–78) | 15 (10–45) | 0.6443 |
| Duration of neutropenia (days) | 15 (2–67) | 16 (3–50) | 14 (6–47) | 14 (6–380) | 0.5182 |
| aGVHD | 168 (39.2%) | 34 (33.0%) | 28 (39.4%) | 28 (48.3%) | 0.3008 |
| cGVHD | 64 (14.9%) | 14 (13.6%) | 10 (14.1%) | 6 (10.3%) | 0.8627 |
| Overall survival | 379 (88.3%) | 86 (83.5%) | 56 (78.9%) | 42 (72.4%) | 0.0047 |
Data are shown as median (range) or n (%). aPatients who received caspofungin or amphotericin as prophylaxis were excluded. bEastern Cooperative Oncology Group score. cOthers include myeloproliferative neoplasms (n=2), solid tumor (n=1), hereditary and metabolic disorders(n=6), unclassified acute leukemia (n=7), chronic lymphocytic leukemia (n=1), Hodgkin lymphoma (n=1) and hemolytic anemia (n=1) in the fluconazole group; multiple myeloma (n=1) in the itraconazole group ; chronic lymphocytic leukemia (n=1), Hodgkin lymphoma (n=1), and chronic myelomonocytic leukemia (n=1) in the voriconazole group; and hereditary and metabolic disorders (n=2) and unclassified acute leukemia (n=1) in the micafungin group. dData were missing for 3 patients in the fluconazole group. aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; IFD, invasive fungal disease.
Most patients (429/661, 64.9%) received fluconazole as the primary prophylaxis, 103 (15.6%) received itraconazole capsules, 71 (10.7%) received voriconazole, and 58 (8.8%) received micafungin. A comparison of these four treatment groups (Table 1) indicated there were significant differences in age, ECOG score, and duration of prophylaxis (P<0.05). In addition, the itraconazole group had the highest cytomegalovirus (CMV) reactivation rate, followed by the fluconazole group, micafungin group, and voriconazole group (P=0.0012). The fluconazole group had the highest survival rate (88.3%), followed by the itraconazole group, voriconazole group, and micafungin group (P=0.0047). However, the four groups had no significant differences in sex, primary disease, acute graft-versus-host disease (aGVHD), chronic graft-versus-host disease (cGVHD), time to neutrophil recovery, time to platelet recovery, and duration of neutropenia.
Occurrence of IFD
A total of 53 patients experienced IFD (5 proven cases and 48 probable cases), corresponding to an overall incidence of 8.01% (Table 2). The median time of IFD onset was 45 days (range: 16 to 75) and the major pathogens were Aspergillus and Candida.
Table 2
| Pathogen | Proven (n=5) | Probable (n=48) | Total (n=53) |
|---|---|---|---|
| Candida spp. | 3 (60.0%) | 4 (8.3%) | 7 (13.2%) |
| Unclassified | – | 1 (2.1%) | 1 (1.9%) |
| C. glabrata | – | 1 (2.1%) | 1 (1.9%) |
| C. krusei | 1 (20%) | – | 1 (1.9%) |
| C. parapsilosis | 1 (20%) | 1 (2.1%) | 2 (3.8%) |
| C. albicans | 1 (20%) | 1 (2.1%) | 2 (3.8%) |
| Aspergillus spp. | 2 (40.0%) | 18 (37.5%) | 20 (37.7%) |
| A. versicolor | – | 1 (2.1%) | 1 (1.9%) |
| A. fumigatus | 1 (20%) | – | 1 (1.9%) |
| Unclassifieda | 1 (20%) | 4 (8.3%) | 5 (9.4%) |
| Unknownb | – | 11 (22.9%) | 11 (20.8%) |
| A. flavus | – | 2 (4.2%) | 2 (3.8%) |
| Candida and Aspergillus spp. | 5 (100%) | 22 (45.8%) | 27 (50.9%) |
a, Belongs to Aspergillus spp., but not A. versicolor and A. fumigatus. The specific category is undefined. b, Belongs to Aspergillus spp., but the specific category is unknown.
Univariate analysis (Table 3) indicated that the factors significantly associated with IFD were donor type (P=0.0013), neutropenia duration of more than 14 days (P=0.0003), persistent fever (P=0.0083), use of a glucosteroid (P=0.0402), Epstein-Barr virus viremia (P=0.0358), cytomegalovirus viremia (P=0.0137), renal insufficiency (P=0.0237), and hypoalbuminemia (P=0.002).
Table 3
| Variable | Total, n (%) | IFD, n (%) | P |
|---|---|---|---|
| Donor typeb | 0.0013 | ||
| Matched sibling | 273 (41.3%) | 10 (3.66%) | |
| Haploidentical | 198 (30.0%) | 24 (12.12%) | |
| Unrelated | 189 (28.6%) | 19 (10.05%) | |
| Neutropenia duration >14 days | |||
| No | 292 (44.2%) | 10 (3.42%) | 0.0003 |
| Yes | 369 (55.8%) | 43 (11.65) | |
| Persistent fever | 0.0083 | ||
| No | 141 (21.3%) | 4 (2.84%) | |
| Yes | 520 (78.7%) | 49 (9.42%) | |
| Use of glucosteroid | 0.0402 | ||
| No | 122 (18.5%) | 4 (3.28%) | |
| Yes | 539 (81.5%) | 49 (9.09%) | |
| Epstein-Barr virus viremia | 0.0358 | ||
| No | 524 (79.3%) | 36 (6.87%) | |
| Yes | 52 (7.9%) | 9 (17.31%) | |
| Untested | 85 (12.8%) | 8 (9.41%) | |
| Cytomegalovirus viremia | 0.0137 | ||
| No | 438 (66.3%) | 26 (5.94%) | |
| Yes | 202 (30.5%) | 26 (12.87%) | |
| Untested | 21 (3.2%) | 1 (4.76%) | |
| Renal insufficiency | 0.0237 | ||
| No | 615 (93.0%) | 45 (7.32%) | |
| Yes | 46 (7.0%) | 8 (17.39%) | |
| Hypoalbuminemia | 0.0020 | ||
| No | 397 (60.1%) | 21 (5.29%) | |
| Yes | 264 (39.9%) | 32 (12.12%) |
aIncludes patients with proven and probable IFD. bData from one patient were missing.
Multivariate analysis indicated that the factors significantly and independently associated with of IFD were neutropenia duration of more than 14 days (aOR: 3.73, 95% CI: 1.66 to 8.36; P<0.001), age greater than 18 years (aOR: 3.37, 95% CI: 1.23 to 9.18; P=0.02), and receipt of transplant from a haploidentical donor (aOR: 5.88, 95% CI: 1.48 to 23.2; P=0.01) or an unrelated matched donor (aOR: 9.03, 95% CI: 2.54 to 32.1; P<0.001) (Table 4).
Table 4
| Factor | P | aOR | 95% CI of OR |
|---|---|---|---|
| Neutropenia duration (>14 days vs. others) | <0.001 | 3.73 | 1.6597–8.3630 |
| Adult vs. childb | 0.02 | 3.37 | 1.2329–9.1841 |
| Haploidentical donor vs. matched sibling | 0.01 | 5.88 | 1.4813–23.322 |
| Unrelated matched donor vs. matched sibling | <0.001 | 9.03 | 2.5366–32.140 |
| Itraconazole vs. Fluconazole | 0.28 | 1.51 | 0.7172–3.1873 |
| Micafungin vs. Fluconazole | 0.38 | 0.56 | 0.1491–2.0794 |
| Voriconazole vs. Fluconazole | 0.26 | 0.41 | 0.0873–1.9537 |
Corrected for confounding by a logistic regression model. aIncludes patients with proven and probable IFD. badult: >18 years old, child: <18 years old.
Effect of different prophylactic drugs on IFD and survival
The overall incidence of probable IFD after transplantation (Table 5) was 7.0% in the fluconazole group, 12.6% in the itraconazole group, 1.4% in the voriconazole group, and 5.2% in the micafungin group (P=0.0379). However, the four groups had no significant differences in the incidence of early IFD, late IFD, or very late IFD (Table 5).
Table 5
| Fluconazole (n=429) | Itraconazole (n=103) | Voriconazole (n=71) | Micafungin (n=58) | P | |
|---|---|---|---|---|---|
| Early IFD (<40 days) | 16 (3.7%) | 3 (2.9%) | – | – | 0.2235 |
| Candida spp. | 2 (0.5%) | – | – | – | |
| Aspergillus spp. | 7 (1.6%) | – | – | – | |
| Unclassified | 7 (1.6%) | 3 (2.9%) | – | – | |
| Late IFD (40 to 100 days) | 11 (2.6%) | 6 (5.8%) | – | 2 (3.4%) | 0.1465 |
| Candida spp. | 1 (0.2%) | – | – | – | |
| Aspergillus spp. | 3 (0.7%) | 5 (4.9%) | – | 1 (1.7%) | |
| Pityrosporum orbiculare | 1 (0.2%) | – | – | – | |
| Unclassified | 6 (1.4%) | 1 (1.0%) | – | 1 (1.7%) | |
| Very late IFD | 3 (0.7%) | 4 (3.9%) | 1 (1.4%) | 1 (1.7%) | 0.0564 |
| Candida spp. | – | – | 1 (1.4%) | – | |
| Aspergillus spp. | 2 (0.5%) | – | – | – | |
| Unclassified | 1 (0.2%) | 4 (3.9%) | – | 1 (1.7%) | |
| Overall IFD | 30 (7.0%) | 13 (12.6%) | 1 (1.4%) | 3 (5.2%) | 0.0379 |
| Candida spp. | 3 (0.7%) | – | 1 (1.4%) | – | |
| Aspergillus spp. | 12 (2.8%) | 5 (4.9%) | – | 1 (1.7%) | |
| Pityrosporum orbiculare | 1 (0.2%) | – | – | – | |
| Unclassified | 14 (3.3%) | 8 (7.8%) | – | 2 (3.4%) |
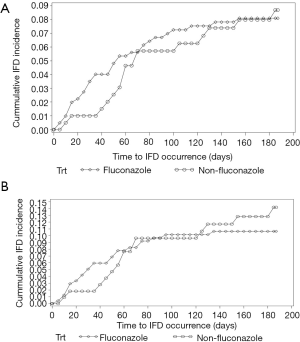
A comparison of fluconazole with non-fluconazole patients indicated similar rates of early IFD in the overall population (Figure 1A) and in the sub-group of patients who received HSCT from haploidentical and unrelated donors (Figure 1B).
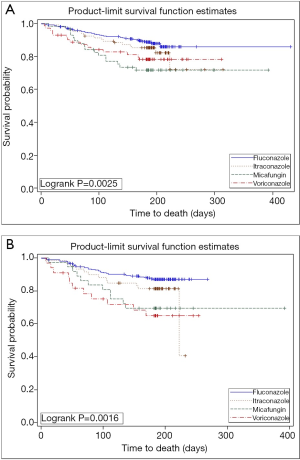
The overall survival rate was 88.3% in the fluconazole group, 83.5% in the itraconazole group, 78.9% in the voriconazole group, and 72.4% in the micafungin group (log-rank test: P=0.0047) (Table 1; Figure 2A). Moreover, subgroup analysis of patients receiving alternative donor transplantation also indicated the fluconazole group had the best overall survival (Figure 2B).
Univariate analysis of factors associated with overall survival (data not shown) indicated the following factors were associated with poor overall survival: advanced disease prior to transplantation (P<0.001), alternative donor type (P=0.002), myeloablative conditioning regimen (P=0.0005), total body irradiation (TBI; P=0.001), oral mucositis (P<0.001), GVHD (P<0.001), persistent fever (P=0.0001), use of broad spectrum antibiotics for more than 7 days (P<0.001), CMV viremia (P=0.0193), renal insufficiency (P<0.001), mechanical ventilation (P<0.001), hypoalbuminemia (P<0.001), and delayed neutrophil engraftment (P<0.001). The final multivariate analysis indicated that the factors significantly and independently associated with poor survival were myeloablative conditioning regimen (P=0.03), TBI (P=0.02), oral mucositis (P<0.001), male sex (P=0.04), advanced disease (P=0.01), and transplant from an unrelated donor (P<0.001) (Table 6).
Table 6
| Risk factor | P | aHR | 95% CI of HR |
|---|---|---|---|
| Age | 0.08 | 1.02 | 0.9981–1.0352 |
| EBV viremia | 0.60 | 0.80 | 0.3426–1.8554 |
| CMV viremia | 0.91 | 0.97 | 0.5461–1.7135 |
| Myeloablative conditioning regimen | 0.03 | 0.50 | 0.2765–0.9204 |
| Total body irradiation | 0.02 | 1.94 | 1.0960–3.4395 |
| Oral mucositis | 0.00 | 2.63 | 1.4799–4.6784 |
| Male vs. female | 0.04 | 1.76 | 1.0295–2.9958 |
| Itraconazole vs. Fluconazole | 0.29 | 1.44 | 0.7279–2.8653 |
| Micafungin vs. Fluconazole | 0.13 | 1.73 | 0.8547–3.5056 |
| Voriconazole vs. Fluconazole | 0.21 | 1.63 | 0.7622–3.5003 |
| Neutrophil engraftment time | 0.02 | 1.07 | 1.0096–1.1400 |
| Platelet engraftment time | 0.71 | 1.00 | 0.9711–1.0202 |
| Advance-stage disease | 0.01 | 0.49 | 0.2910–0.8414 |
| Complicated with GVHD | 0.95 | 1.02 | 0.6139–1.6785 |
| Haploidentical donor vs. matched sibling | 0.28 | 1.51 | 0.7101–3.2126 |
| Unrelated matched donor vs. matched sibling | <0.001 | 2.89 | 1.5531–5.3807 |
aCorrected for confounding by Cox proportional hazard model.
Discussion
The present study results demonstrated that the efficacy of fluconazole is similar to that of mold-active drugs in preventing early IFD among allo-HSCT transplant recipients when the donors were matched siblings or alternative donors. This suggests that fluconazole remains useful in preventing early IFD, in line with European guidelines for primary antifungal prophylaxis in adult haematology patients (22). Thus fluconazole is recommended during pre-engraftment based on AI evidence, and the other three drugs (itraconazole, voriconazole and micafungin) are recommended based on BI evidence.
Clinicians now commonly use mold-active drugs as prophylaxis for IFD after allo-HSCT, because the dominant IFD pathogen in patients receiving HSCT has shifted from Candida to Aspergillus (3,10-12). However, the usefulness of mold-active drugs for IFD prophylaxis is still controversial. Several prospective randomized studies have compared fluconazole with other mold-active drugs, but the results were inconsistent. Most of these studies failed to demonstrate the superiority of mold-active drugs over fluconazole. One open-labeled multicenter randomized trial (13) that compared itraconazole (200 mg intravenously every 12 h for 2 days followed by 200 mg intravenously every 24 h or a 200-mg oral solution every 12 h) with fluconazole (400 mg intravenously or orally every 24 h) from day 1 until day 100 after transplantation showed that itraconazole led to fewer proven cases of IFD (9% vs. 25%, P=0.01). However, another randomized study (17) compared fluconazole (400 mg/day) and itraconazole (oral solution or intravenous) for 180 days after HSCT and the results indicated similar proven or probable IFD cases in the two arms (fluconazole 16% vs. itraconazole 13%, P=0.46). Another prospective randomized study comparing voriconazole and fluconazole (14) also demonstrated that the incidence of IFD was similar in patients receiving voriconazole and fluconazole for 100 days (or 180 days for high-risk patients). A large randomized study (15) compared micafungin and fluconazole for IFD prophylaxis during neutropenia in HSCT patients and showed that prophylaxis with micafungin was associated a lower incidence of IFD. Another randomized study (16) compared micafungin and fluconazole (initiated within 24 h of HSCT and maintained for up to 21 days), and found that the incidence of proven and probable IFD cases within 100 days and the mortality at 100 days were similar in the two groups.
In contrast to the present study, none of these previous studies distinguished early, late, and very late IFD after HSCT. The timing of IFD is important because patients experience changes in immune status and predisposing factors over time. In particular, invasive Candida tends to occur soon after allo-HSCT (mainly due to neutropenia and mucosal injury) whereas invasive Aspergillus tends to occur long after allo-HSCT (mainly due to GVHD) (23). Aspergillus and Candida are the predominant pathogens after allo-HSCT. Aspergillus produces spores that are present in ambient air and patients acquire infection through inhalation from respiratory tract, resulting in pulmonary infection (24). Candida colonizes the upper respiratory tract, gastrointestinal tract, urogenital tract, and other regions (25). When mucosal membrane barriers are disrupted, this pathogen invades adjacent tissues and disseminates to other organs. During the early phase after allo-HSCT, patients are cared for in HSCT wards that have laminar flow, and thus are not susceptible to Aspergillus. However, Candida species is the major pathogen responsible for infections during the early phase, due to breaks in the mucocutaneous barriers as a result of conditioning regimens (26). Hence, we speculate that Candida is the most prevalent pathogens during the early phase, and that fluconazole is as effective as anti-mold agents in preventing early IFD after allo-HSCT. However, no randomized studies have yet focused on the timing of IFD after HSCT. To the best of our knowledge, only two studies (15,16) focused on the neutropenic phase; these studies compared micafungin and fluconazole but presented inconsistent results. Therefore, the most recent recommendations are that the selection of prophylactic treatment depends on the time after HSCT and on the other risk factors present in individual patients (19,20).
Because the risk of IFD is associated with the type of transplantation, one of our interesting results is that fluconazole was effective for haploidentical recipients and HLA-matched recipients. Our previous studies (3-5) of patients undergoing haploidentical or unrelated donor transplantation indicated the incidence of IFD was significantly greater than in patients undergoing matched sibling donor transplantation. Although several mold-active drugs are recommended for such patients, the optimal prophylaxis for patients undergoing haplo-HSCT remains to be determined. The GITMO guidelines (19) recommend an anti-mold agent for such patients, but the evidence supporting this recommendation is not strong. A small retrospective study (27) from France compared micafungin, fluconazole, and itraconazole in 99 patients who underwent haplo-identical HSCT and received post-transplant cyclophosphamide. As expected, the results demonstrated micafungin had better efficacy than fluconazole in preventing invasive aspergillosis in these patients. These researchers also demonstrated that when considering all fungal infections, micafungin was more effective than itraconazole in preventing all IFD episodes. These results are in contrast with those of the present study. We can suggest several reasons for this discrepancy. First, the French study enrolled only 99 patients, and it was not prospective. Second, almost all patients with haploidentical donors in the CAESAR study used the “Beijing Protocol”, which has important differences from haploidentical SCT using post-transplant cyclophosphamide. It is still unknown whether there are differences in immune reconstitution for patients receiving these different methods. Therefore, future prospective studies are needed to examine this issue, because this is an increasingly common transplant platform.
There were some limitations to the present study. First, this is an observational study, and the prescriptions of prophylactic drugs were highly heterogeneous in terms of dosing, duration, and timing. Even with this limitation, our study provides valuable information, because it includes all drugs available in a real world setting that are used for IFD prophylaxis. In addition, our study population had a large proportion of haploidentical HSCT recipients, obtained using a unique protocol. Second, because the median duration of prophylaxis in our study was approximately 30 days, we have no information allowing comparison of mold active-drugs with fluconazole during the GVHD phase. Other researchers have recently developed a risk-based prophylaxis strategy (28), which seems to be highly attractive. Several factors, including donor source, history of IFD, presence of active hematologic cancer, and GVHD, may help identify patients with increased risk of IFD throughout the post-HSCT period, and might help clinicians to develop individualized prevention strategies. However, whether and how risk factors and time after HSCT can be incorporated into risk calculation is still unclear, and this topic requires further investigation. Finally, this study was performed before posaconazole was available in the China mainland, and we therefore did not have data comparing fluconazole with posaconazole. A recent study that compared posaconazole with fluconazole during the GVHD phase after allo-HSCT found that posaconazole was associated with a lower incidence of IFD and improved overall survival (29). However, no such study has yet compared different prophylaxes during the early phase (neutropenia) after transplantation.
Conclusions
In conclusion, the results of the present study suggest that fluconazole can be a good choice for prevention of early IFD following allo-HSCT, even in high-risk patients undergoing transplantation from alternative donors. Further prospective randomized studies are necessary to confirm this conclusion.
Acknowledgments
We thank all the participating centers who contributed to data collection. This manuscript has been edited and proofread by Medjaden Bioscience Limited.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2887
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2887
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2887). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Peking University, People’s Hospital (NO. V20100812) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation (first of two parts). N Engl J Med 1975;292:832-43. [Crossref] [PubMed]
- Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010;363:2091-101. [Crossref] [PubMed]
- Sun Y, Meng F, Han M, et al. Epidemiology, management, and outcome of invasive fungal disease in patients undergoing hematopoietic stem cell transplantation in China: a multicenter prospective observational study. Biol Blood Marrow Transplant 2015;21:1117-26. [Crossref] [PubMed]
- Sun Y, Xu L, Liu D, et al. Incidence of invasive fungal disease after unmanipulated haploidentical stem cell transplantation was significantly higher than that after HLA-matched sibling transplantation. Clin Microbiol Infect 2013;19:1029-34. [Crossref] [PubMed]
- Sun YQ, Xu LP, Liu DH, et al. The incidence and risk factors of invasive fungal infection after haploidentical haematopoietic stem cell transplantation without in vitro T-cell depletion. Clin Microbiol Infect 2012;18:997-1003. [Crossref] [PubMed]
- Gao L, Sun Y, Meng F, et al. Antifungal prophylaxis of patients undergoing allogenetic hematopoietic stem cell transplantation in China: a multicenter prospective observational study. J Hematol Oncol 2016;9:97. [Crossref] [PubMed]
- Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992;326:845-51. [Crossref] [PubMed]
- Chang YL, Yu SJ, Heitman J, et al. New facets of antifungal therapy. Virulence 2017;8:222-36. [Crossref] [PubMed]
- Rauseo AM, Coler-Reilly A, Larson L, et al. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect Dis 2020;7:ofaa016.
- Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010;50:1091-100. [Crossref] [PubMed]
- Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 2009;48:265-73. [Crossref] [PubMed]
- Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study--Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis 2007;45:1161-70. [Crossref] [PubMed]
- Winston DJ, Maziarz RT, Chandrasekar PH, et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med 2003;138:705-13. [Crossref] [PubMed]
- Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010;116:5111-8. [Crossref] [PubMed]
- van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004;39:1407-16. [Crossref] [PubMed]
- Park S, Kim K, Jang JH, et al. Randomized trial of micafungin versus fluconazole as prophylaxis against invasive fungal infections in hematopoietic stem cell transplant recipients. J Infect 2016;73:496-505. [Crossref] [PubMed]
- Marr KA, Crippa F, Leisenring W, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood 2004;103:1527-33. [Crossref] [PubMed]
- Boga C, Bolaman Z, Cagirgan S, et al. Recommendations for Risk Categorization and Prophylaxis of Invasive Fungal Diseases in Hematological Malignancies: A Critical Review of Evidence and Expert Opinion (TEO-4). Turk J Haematol 2015;32:100-17. [Crossref] [PubMed]
- Girmenia C, Barosi G, Piciocchi A, et al. Primary prophylaxis of invasive fungal diseases in allogeneic stem cell transplantation: revised recommendations from a consensus process by Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant 2014;20:1080-8. [Crossref] [PubMed]
- Pagano L, Busca A, Candoni A, et al. Risk stratification for invasive fungal infections in patients with hematological malignancies: SEIFEM recommendations. Blood Rev 2017;31:17-29. [Crossref] [PubMed]
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- Maertens JA, Girmenia C, Bruggemann RJ, et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother 2018;73:3221-30. [Crossref] [PubMed]
- Person AK, Kontoyiannis DP, Alexander BD. Fungal infections in transplant and oncology patients. Infect Dis Clin North Am 2010;24:439-59. [Crossref] [PubMed]
- Lopez-Medrano F, Fernandez-Ruiz M, Silva JT, et al. Clinical Presentation and Determinants of Mortality of Invasive Pulmonary Aspergillosis in Kidney Transplant Recipients: A Multinational Cohort Study. Am J Transplant 2016;16:3220-34. [Crossref] [PubMed]
- Trief D, Gray ST, Jakobiec FA, et al. Invasive fungal disease of the sinus and orbit: a comparison between mucormycosis and Aspergillus. Br J Ophthalmol 2016;100:184-8. [Crossref] [PubMed]
- Natesan SK, Chandrasekar PH. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: current evidence, safety, efficacy, and clinical recommendations. Infect Drug Resist 2016;9:291-300. [Crossref] [PubMed]
- El-Cheikh J, Crocchiolo R, Vai A, et al. Comparison of Three Distinct Prophylactic Agents Against Invasive Fungal Infections in Patients Undergoing Haplo-identical Hematopoietic Stem Cell Transplantation and Post-transplant Cyclophosphamide. Mediterr J Hematol Infect Dis 2015;7:e2015048. [Crossref] [PubMed]
- Wang L, Wang Y, Hu J, et al. Clinical risk score for invasive fungal diseases in patients with hematological malignancies undergoing chemotherapy: China Assessment of Antifungal Therapy in Hematological Diseases (CAESAR) study. Front Med 2019;13:365-77. [Crossref] [PubMed]
- Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007;356:335-47. [Crossref] [PubMed]


