Vitamin D modulation and microRNAs in gastric cancer: prognostic and therapeutic role
Introduction
Gastric cancer (GC) is the forth most common cancer and the third cause of cancer mortality (1), although its incidence and mortality rates have been declining steadily since last century, in part due to a change of dietary habits and lifestyle, better conditions in food conservation and eradication of Helicobacter pylori (H. pylory) (1-4). However, this decrease in GC incidence and mortality is explained by a lower incidence of distal tumors, finding that proximal and junctional tumors have increased their incidence (5).
Data from many ecologic studies support a strong association between sun exposure and GC incidence and mortality (6). However, studies on vitamin D intake and its serum status and their association with risk and mortality of GC, show more variable data (6).
Adenocarcinoma is the most common histology and this review focuses on this subtype (7). Also tumor location (proximal vs. distal tumors; cardia vs. no cardia) should be consider in treatment decisions.
Although surgical resection with lymphadenectomy is considered the only curative therapy for GC, multimodality treatment has become the standard of care for GC staged as IB-III (8,9). Adjuvant chemotherapy and chemoradiation, as well as perioperative chemotherapy are treatment approaches that have reduced recurrence rate and increased survival (10-16). After curative treatment, relapse is the most dreadful event, which rarely has a curative option (17,18). Five year survival rate in metastatic GC is only 4%. In this setting, chemotherapy based on Cisplatin (CDDP), 5-Fluorouracil (5FU), Taxanes, Irinotecan, Trastuzumab (antiHER2 therapy) and in subsequent lines, Ramucirumab and immune checkpoint inhibitors, has improved median overall survival achieving 11–13 months (19).
miRNAs are endogenous small non-coding RNA molecules of 18–25 nucleotides that function at postranscriptional level, inhibiting translation or promoting degradation of messenger RNA (mRNA) with complementary sequences. Thus, miRNAs regulate the production of hundreds of different proteins, including transcription factors (20-22).
This is not the first review attempting to collect current evidence on Vitamin D modulation of miRNAs expression in cancer (23,24). But in this review we have tried to update the evidence on several miRNAs with prognostic value and therapeutic potential in GC, whose expression may be influenced by vitamin D or may regulate vitamin D signaling.
Prognostic factors in GC. Histology and molecular classifications as prognostic and predictive factors in GC
The TNM staging system is the most relevant prognostic factor and a tool commonly used in clinical practice for setting cancer stage-specific therapeutic strategy (25).
Most existing data on prognostic factors are based on treatment with surgery alone. There are several prognostic indices for GC and nomograms for survival after R0 resection (R0 indicates a microscopically margin-negative resection) (26). More recent nomograms include factors as age at diagnosis, sex, tumor location, depth of invasion, number of metastatic lymph nodes, number of examined lymph nodes, lymphatic/venous invasion and Lauren classification as covariates associated with prognosis (17,27).
In a recent systematic review and meta-analysis, van den Ende et al. identified 23 potentially relevant prognostic and 15 predictive factors for the curative treatment of GC (28).
According to Lauren classification, gastric adenocarcinomas are subdivided into intestinal and diffuse subtypes (29). Furthermore, a genomic classification identified molecular types of GC, intestinal (G-INT) and diffuse (G-DIF), stratified into prognostic groups, with a worse prognosis for the diffuse subtype and associated with worse response to chemotherapy (30).
The molecular definition of the diffuse subtype is loss of E-cadherin expression (31). In contrast, carcinogenesis of the intestinal type follows a multi-step process, generally initiated by H. pylori infection and sequential phases of non-atrophic active chronic gastritis, multifocal atrophic gastritis, intestinal metaplasia, dysplasia and invasive carcinoma (32).
The role of histology as a prognostic and predictive factor of response to chemotherapy is a topic of interest. A meta-analysis showed that histology, according to Lauren classification, might be a useful prognostic marker for both early and advanced GC patients, with intestinal-type associated with better outcome (33). More evidence from observations of real-world data supports that tumor subtypes in Lauren classification can predict survival and different responses to chemotherapy (34).
Although expression of miRNAs related to histological subtypes of GC has not been widely analyzed, Yepes et al. showed that miR-100, miR-125b, miR-99a and let-7c were associated with diffuse subtype (35).
Among different attempts, two molecular classifications are worth mentioning. The Cancer Genome Atlas (TCGA) investigators published one comprehensive study on molecular GC classification (36) and four molecular subtypes were identified: EBV (Epstein Barr virus)-related in 9% of cases, MSI-H (microsatellite instability-high) in 22% of cases, GS (Genomically Stable) in 20% of cases and CIN (Chromosomal Instability) in 50% of cases.
Based on this classification, a prediction model was reported by Sohn et al. (37), showing best prognosis for EBV subtype, an intermediate prognostic group integrated by MSI-H and CIN subtypes, and the GS as the subtype with the worst prognosis. Also, they reported an association between CIN subtype and benefit from adjuvant chemotherapy, with the limitations of retrospective analysis (37).
The Asian Cancer Research Group (ACRG) provided another GC classification and identified also four subtypes (38). The MSI (Microsatellite unstable) subtype, in 23% of cases, associated with the best overall prognosis, antrum, hyper-mutated intestinal subtype, and early stage. The MSS/EMT (Epithelial to mesenchymal transition) group, in 15% of cases, associated with the worst prognosis, diffuse histology and advanced stage. The MSS/TP53 active subtype, in 26% of cases, showed EBV positivity and preserved activity of TP53. After MSI subtype, had the best overall prognosis. And MSS/TP53 inactive subtype, in 36% of cases, with the highest prevalence of TP53 mutations, amplifications of tyrosine kinasse receptors and an intermediate prognosis (38,39).
Recently, miR-338/CCL21/NF-kB signaling, miR-146b/PSMD3/proteasome, miR-34a/VCL/focal adhesión and miR-34c/VCL/focal adhesion subtype-specific subpaths were identified and might have a role in GC development and classification, although data on their prognostic value have not been published (40).
Vitamin D and cancer
Approximately 3 percent of the human genome is under control of vitamin D (41).
Vitamin D is obtained from foods and ultraviolet–B radiation exposure in the skin, through convertion of 7-dehydrocholesterol that is a prohormone that needs two hydroxylation steps, through two enzimes of cytochrome P450, to be active. Firstly, vitamin D is converted to 25-hydroxyvitaminD3 (25(OH)D) by 25-hydroxylase (CYP27A1) in the liver. Then, in the kidney, is converted to 1-alpha,25 dihidroxyvitaminD3 (Calcitriol), its active form, by 1-alpha-hydroxylase (CYP27B1) (42). To facilitate reading, we will continue to use the term vitamin D as an equivalent among its metabolites.
There is some evidence that several tissues, outside the kidney, can generate this active form, in an paracrine (or autocrine) way, by expressing 1-alpha-hydroxilase (41,43).
The vitamin D receptor (VDR) belongs to the superfamily of steroid/thyroid hormone receptor and is nearly universally expressed in nucleated cells (41). In the absence of ligand, some basal level of receptor remains in the nucleus associated with co-repressor complex and leads to silencing of transcription (44).
The union of calcitriol with VDR induce a dimerization with the retinoid X receptor (RXR) and then the complex calcitriol-VDR-RXR translocates into the nucleus. This complex attaches to the vitamin D response elements (VDREs) in the promoters of target genes determinants in the pathways of proliferation, apoptosis and angiogenesis (42). This signal is limited in time, conditioned by the rapid metabolism of the ligand (CYP24A1) and the proteasome mediated receptor degradation (41,44).
The role of vitamin D and its receptor (VDR) in cancer incidence and mortality have been studied for long time (6,45-48). It is suggested that vitamin D deficiency might increased both (49).
In cancer prevention, the majority of vitamin D intervention trials do not show a reduction in cancer risk, however most of them have been carried out in adults without vitamin D deficiency (50-55). Furthermore, it has been reported that common polymorphisms in the VDR modified the association of serum vitamin D and that the relationship of low serum vitamin D levels with major health outcomes varied based on these common genetic differences (56,57).
In a recent randomized trial of postoperative vitamin D3 supplementation, double-blind placebo control carried out in Japan, in patients with digestive tract cancers, staged I-III, 417 patients were recruited, 42% with GC. Subgroups were made based on baseline serum vitamin D levels (0–20; 20–40; >40 ng/mL) and they reported no significant improvement in relapse free survival (RFS) at 5 years (58). Nevertheless, the authors, in a post hoc analysis, found that the use of bioavailable 25(OH)D, rather than total 25(OH)D, as a biomarker of vitamin D deficiency, indicated a significant difference in RFS at 5 years in the subgroup of patients with low bioavailable 25(OH)D levels (77% in the vitamin D group versus 58% in the placebo group) and no differences in the subgroup with high bioavailable levels of 25(OH)D, suggesting a role for supplementation in patients with bioavailable 25(OH)D deficiency (59).
In a retrospective case-control study, a higher prevalence of vitamin D insufficiency and deficiency was found in GC patients compared to controls without known malignancy (60). In another case-control study designed to evaluate the risk of GC development related to VDR and VDBP (VD binding protein) polymorphisms and Vitamin D serum levels, the authors noted that GC patients had lower vitamin D levels than controls, and found a fourfold higher risk of GC development in cases with severe vitamin D deficiency (<10 ng/mL) (61). Furthermore, in GC clinical stage and lymph node metastasis were found to be inversely correlated with vitamin D levels. Therefore, vitamin D level was proposed as an independent prognostic factor, with a worse prognosis associated with vitamin D deficiency (62).
In vitro, Bao et al. reported that direct administration of vitamin D in GC cells induced apoptosis and enhanced VDR, CYP24A1 and p21 expression levels (63).
In a subsequent study, they treated GC cells with vitamin D or CDDP alone or a combination of both and showed that vitamin D potentiated the effects of CDDP, showing upregulation of BAX, a decrease in levels of phosphorylation of ERK and AKT and a positive regulation of p21 and p27, which induced the cells cycle arrest in G0/G1 phase (64).
Furthermore, it has been suggested that vitamin D acts through the Hedgehog signaling pathway and regulates the expression of PTEN (65) and promotes apoptosis in undifferentiated GC cells (62).
Interestingly, Han et al. conducted a multicenter prospective cohort study to determine whether serum vitamin D level had an impact on H. pylori infection (Main causal agent of GC) and eradication, showing that serum vitamin D level of ≥10 ng/mL was an independent risk factor for a successful H. pylori eradication (66).
MicroRNAs
Bioinformatic analysis estimated that more than 60% in mammalian genome could be targeted by single miRNA (67).
In a must-read article, Bartel explained that in the canonical biogenesis of miRNAs, in animals, miRNAs are transcribed by RNA polymerase II (Pol II) as a longer precursor “pri-miRNA”, which is processed by a complex formed by Drosha endonuclease and DGCR8, known as Microprocessor (68). Drosha cut one strand of the stem of the pri-miRNA hairpin and liberates a 60 nucleotides stem-loop called “pre-miRNA” that is exported to the cytoplasm through the action of Exportin 5 and RAN-GTP. In the cytoplasm, the pre-miRNA is processed by Dicer, another endonuclease also associated with TRBP. Dicer cuts both strands near the loop and generates a miRNA duplex. The miRNA and its passenger strand, as a duplex is loaded into an Argonaute protein (Ago). After the expulsion of the passenger strand, the mature silencing complex is form (RISC) (68). Once loaded into the silencing complex, most miRNAs are very stable, with half-lives of days (68). The passenger strand is thought to be degraded, although there is evidence that mature miRNA and its passenger strand can work in a coordinated manner, improving their repressive effect by binding to the same target (69).
In humans, the repression mode acts without slicing the mRNA. Ago recruits TNRC6 and interacts with the mRNA and causes its destabilization (68).
miRNAs are grouped into families based on their targeting properties, which depend primarily on the identity of their extended seed region (miRNA nucleotides 2–8) (68).
Prognostic and potential therapeutic role of miRNAS in GC
In a systematic review of the GC miRNA expression profile, 352 miRNAs were found differentially expressed when tumor tissue was compared to normal gastric tissue, highlighting the upregulation of miR-21, as the most consistent finding (70).
In another review, a comparison of 14 miRNAs in blood and 36 in tissues of GC patients was made according to their association with clinicopathological characteristics and survival (71). They showed that 42.86% of blood miRNAs have a significant association with one or two clinicopathological characteristics, although around 60% of miRNAs in tissues were significantly correlated to more than three clinicopathological features (71).
Furthermore, upregulation of Drosha, Dicer and DGCR8 was observed in GC tissues compared to normal marginal gastric tissues, although no correlation to clinicopathological characteristics was found, but probably influencing in deregulation of other miRNAs (72).
miRNAs are frequently localized to cancer-linked genomic regions, as well as deregulated in tumors versus normal tissues (73,74). Moreover, miRNAs can regulate oncogenes and tumor suppressors, even acting as such themselves. All this, added to their stability in many body fluids (67) and their role as diagnostic and prognostic biomarkers, give miRNAs great therapeutic potential.
miRNAs could be therapeutic targets, therapeutic weapons, and response prediction tools for other treatments. As pointed out by Tsai et al. methods currently under development are anti-miRNA oligonucleotides, miRNA sponges, miR-Mask, antagomiRs, and miRNA inhibitors (75).
Some oligonucleotide drugs approved induce cleavage of a target mRNA or modulate the splicing pattern (56). Some miRNA inhibitors also are in clinical development (76,77), but safety and delivery of these drugs remains a challenge.
Otherwise, chemotherapy and targeted therapy resistance are challenging in GC. Several miRNAs have been implicated in multidrug resistance (MDR) (78). Moreover, it has been shown that vitamin D can potentiate the effects of chemotherapy and contribute to overcome the molecular mechanisms of drug resistance (79).
In our review line, we will discuss of each proposed miRNA the way in which vitamin D modulates it, as well as the data in favour of its prognostic and therapeutic role in GC (Table 1).
Table 1
| miRNAs related to vitamin D | Potential role in GC | Expression in GC | Therapeutic potential in GC | Reference |
|---|---|---|---|---|
| miR-125 | Oncogene | Upregulated | Possible role in CDDP sensitivity and Trastuzumab resistance in GC cells | (80-83) |
| TS | Downregulated | |||
| miR-145 | TS | Downregulated | Possible role improving chemosensitivity to CDDP and 5FU in GC cells | (84,85) |
| miR-99 | Oncogene | Upregulated | Associated with CDDP resistance in GC cells | (86,87) |
| TS | Downregulated | |||
| miR-498 | TS | Downregulated | Possible role in overcoming CDDP resistance in GC cells | (88,89) |
| miR-22 | TS | Downregulated | Possible role in CDDP resistance in GC cells | (90-92) |
| miR-181 | Oncogene | Upregulated | – | (93-95) |
| TS | Downregulated | |||
| miR-106 | Oncogene | Upregulated | – | (96,97) |
| miR-532 | Oncogene | Upregulated | – | (98,99) |
| TS | Downregulated |
GC, gastric cancer; miR, microRNA; TS, tumor suppressor; CDDP, cisplatin; 5FU, 5-fluorouracil.
Regulatory micrornas of vitamin d signalling linked to GC
miR-125b
miRNA-125b (miR-125b) is an ubiquitous miRNA and is deregulated in different tumors, although is upregulated in colon and hematopoietic tumors and downregulated in others (Hepatocellular) (100). miR-125a and miR-125b share the same seed region, suggesting they regulate the same target mRNAs (100). Theorically, it has been hypothesized that its targets are tissue and tumor specifics, suggesting that its function depends on the levels of target gene expression (100).
Mohri et al. showed that miR-125b prevents the antiproliferative effects of 1,25 (OH)D3, through posttranscriptional regulation of VDR (101). In cancer cells, they showed that endogenous VDR level was repressed by miR-125b and identified a potential miR-125b recognition element (MRE125b) on VDR mRNA (101).
Additionally, a new study indicated that miR-125b regulates the CYP24 mRNA, recognizing the MRE125b on the CYP24 mRNA (102).
The level of VDR expression has been reported to be a good prognostic factor in breast cancer (103) and esophageal adenocarcinoma (104), as well as the lowest levels of VDR expression occurred in tumor tissue compared to premalignant lesions of GC (105) and of cholangiocarcinomas (106).
In parallel, a progressive downregulation of miR-125a-5p and miR-125b was observed in gastric samples from normal mucosa, to premalignant lesions and finally to intestinal-type adenocarcinoma (80). It has been reported that miR-125b was frequently downregulated in GC tissues (81,82,107) as well as in GC cell lines (82,107). The qRT-PCR levels of miR-125a-5p and miR-125b were inversely correlated with HER2 expression (80). Also, HER2 amplification significantly increased from premalignant lesions to carcinoma and the expression levels of miR-125a-5p and miR-125b were significantly lower in HER2-positive GC than in HER2-negative ones (80). HER2 status is a predictive biomarker commonly used in clinical practice to determine benefit of trastuzumab in combination with first line chemotherapy for metastatic GC (108).
Moreover, HER2 was indicated as a direct target of miR-125b in GC cell lines and miR-125b overexpression prevented proliferation, migration and invasion, in vitro and in vivo and enhanced CDDP sensitivity in GC cells (82). Clinical features associated with miR-125b were gender, lymph node metastasis and HER2 status. Patients with high expression of miR-125b had better progression free and overall survival (82). In another study, miR-125b-2 mimic transfection in GC cells reduced cell viability, migration and invasion and increased cell death, targeting PIK3 (107). In contrast, it has been suggested a possible role of miR-125b in trastuzumab resistance mechanisms, what is of tremendous interest in clinical practice (83). GC patients HER2 positive treated with trastuzumab, showed worse overall survival when they had high miR-125b expression (83).
Wu et al. found in GC patients that miR-125b levels were inversely correlated with invasion depth, clinical stage, and lymph node metastasis. Moreover, primary tumors with low expression of miR-125b had a shorter overall and disease free survival (81). miR-125b targeted MCL1 (myeloid cell leukemia 1), thereby suppressing proliferation and favouring apoptosis. Aditionally, they transfected GC cells with miR-125b and showed sensitization to 5FU-induced apoptosis (81).
In contrast, miR-125b overexpression was observed in GC tissues compared to matched normal tissues (83,109-111) and associated with T stage, lymph node metastasis, TNM stage and worse overall survival (83,110,111). miR-125b has been linked to enhanced cellular proliferation and decreased apoptosis (109).
In the same way, it was suggested that miR-125b could act as an oncogene by targeting PPP1CA and up-regulating Rb phosphorylation in GC (110) and that miR-125b overexpression induced cell migration and invasion, through targeting STARD 13 and NEU1 (111). Additionally, miR-125b is upregulated by KDMB4, which has been linked to gastric carcinogenesis and mediate KDM4B induced activation of Wnt signaling (112).
MicroRNAs regulated by vitamin D and linked to GC
miR-145
Downregulation of miR-145 has been reported in GC and associated with unfavourable prognosis in various tumors, including GC (84,113-116). Recently, miR-145 has been suggested as a marker of cancer migration and invasion in differents malignant tumors (117).
miR-145 expression level has been found downregulated in GC tissues and cell compared to normal (85,115,118) and associated with tumor depth, lymph node metastasis, lymphatic invasión and TNM stage (85).
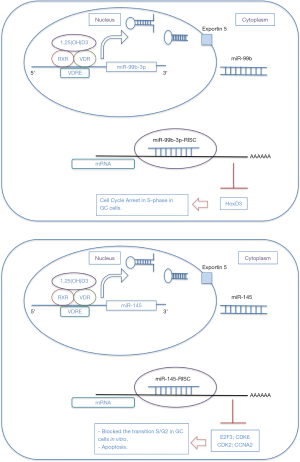
Chang et al. after treating GC cells with vitamin D3, found a decreased percentage of cells in phase S and promoted apoptosis, as well as an increased in miR-145 expression level (115). They determined that inhibition of miR-145 led to a decreased in the antiproliferative effects of vitamin D. Furthermore, they indicated that vitamin D3-induced increase in miR-145 expression was mediated by VDR. In vitro, miR-145 brought about a blockage in S phase/G2 transition in GC cells and suppressed at protein level both E2F3 and CDK6, and downregulated CDK2 and CCNA2 (Figure 1) (115).
Findings in another report suggested that miR-145 might have a role as a tumor suppressor and examined other potential targets whose expression could be modulated by miR-145 in GC cells, such as downregulation of C-MYC, PI3K/AKT, MMP2 and MMP9 proteins and upregulation of p21 (116).
After performing miRNA microarray profiling in GC, precursor lesions and normal tissues, to find differentially expressed miRNAs during early gastric carcinogenesis, Hwang et al. found that downregulation of miR-145 might be an early event in the development of premalignant gastric lesions. In comparison to normal gastric mucosa, adenomas showed decreased expression in five miRNA (miR-26a, miR-375, miR-574-3p, miR-145 and miR-15b) (120).
Interestingly, in GC cells, overexpression of miR-145 inhibited migration, invasion and downregulated ZEB2 and N-Cadherin and increased E-cadherin expression, by direct regulation of ZEB2 (85).
Similarly, Gao et al. observed that N-cadherin protein expression was elevated in GC tissues samples with low levels of miR-145 (118). Here too, miR-145 suppressed GC cell invasion and migration, in vitro and in vivo. In vitro, GC cells expressing miR-145 could reduce N-cadherin levels. In vivo, miR-145 positive tumors were less locally invasive and miR-145 prevented the spread of lung metastases (118).
In this line of research, Xing et al. demonstrated that CTNND1 (Catenin-δ1) is a direct target of miR-145. CTNND1 is part of the cadherin-catenin complex, bound to the cytoplasmic tail of E-cadherin and associated with cell migration, invasion and cell division (121). In vitro, miR-145, through its downregulation, mediated CTNND1 translocation from cytoplasm to membrane, recovering the membranous localization of CTNND1 and E-cadherin (121). CTNND1 promoted GC cell migration and invasion, with no effects on proliferation and apoptosis. They also indicated that cytoplasmic localization of CTNND1 protein was associated with higher clinical stage, positive lymph node metastasis and poorer prognosis in GC patients (121).
Additionally, in GC cells miR-145 directly targeted FSCN1 and an inverse correlation between expression level of miR-145 and FSCN1 in normal/tumor paired samples of GC patients was observed (122). According to this finding, it was possible to distinguish between invasive and expansive GC based on the fact that the former had lower levels of miR-145 and higher levels of FSCN1 (122). Fascins are actin-binding proteins that stimulates cell motility, migration and invasion (122) and FSCN1 is absent in normal epithelia, but overexpressed in several carcinomas (123).
Likewise, downregulation of miR-145 in GC tissues compared to normal gastric mucosa, was inversely correlated with v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets1) overexpression in GC tissues and in GC cell lines. Ets1 overexpression was associated with deeper gastric wall invasion, lymph node metastasis, distant metastasis and advanced TNM stage (124).
Ets1 is a transcription factor linked to migration, invasion and angiogenesis of cancer cell and is a direct target of miR-145 in GC cells (124), causing, in vitro and in vivo, inhibition of invasion, metastasis and angiogenesis.
Furthermore, Zeng et al. published interesting results on the prognostic and therapeutic potential of miR-145 (84).
CD44 (Cluster of differentiation 44)-positive GC cells shows self-renewal abilities and chemoresistance, as stem cells do and its expression is associated with aggressive tumor phenotype (125) and it is suggested that miR-145 is a key regulator of stem cell properties in GC cell by targeting the CD44 3´UTR (84). They demonstrated that repression of CD44 is necessary for miR-145 inhibition of self-renewal properties of GC cells (84). After administration of CDDP and 5FU to GC cells, miR-145 improved chemosensitivity and overexpression of CD44 was associated with resistance to these drugs. However, the miR-145-mediated chemosensitivity can be overcome by re-expression of CD44. Thus, they analyzed that miR-145 mimic treatment of GC cells repressed ABCG2 expression [an ABC transporter involved in drug efflux and a marker of GC stem cells (126)] and that CD44 overexpression enhanced ABCG2 expression (84).
Besides, tumor spheres used in their experiments expressed higher levels of SOX2, OCT-4 and Nanog, which demonstrated that spheres enriched the GC stem cells population (84). Interestingly, SOX2, OCT-4 and Nanog are transcription factors responsible of regulation of self-renewal and pluripotency of stem cells (127). Moreover, in human embryonic cells, miR-145 is upregulated during differentiation and represses OCT-4 and SOX2 expression, inhibits their self-renewal properties, prevents expression of pluripotency genes and induces lineage-restricted differentiation (128) and finally, OCT-4 can repress miR-145 promoter, suggesting a double negative feedback loop. More recently, it has been reported overexpression of SOX2, OCT-4 and NANOG in GC tissues compared to paired normal gastric tissues and association with T size, TNM, tumor grade and worse survival (129).
miR-99 family
Recently, miR-99b-3p was found underexpressed in GC tissues compared to normal and linked to inhibition of cell viability and induction of cell cycle arrest in S-phase in GC cells, by targeting HoxD3 (119). As well, miR-99b-3p was induced by vitamin D through direct VDR binding on its promoter domain (Figure 1) (119).
miR-99 family is composed by miR-99a, miR-99b and miR-100. Their target genes include mTOR, Homebox A1 (HOXA1), CTD small phosphatase-like, N-myristoyltransferase 1 (NMT1), Transmembrane protein 30a (TMEM30A) and SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 5 (SMARCA5) (130).
miR-99b-5p and miR-99a were reported underexpresed in GC tissues (86,131) and cells (86). Furthermore, both exert their effects through targeting IGFR1 (86,131). miR-99b-5p expression level was associated with suppression of cell growth, colony formation, cell cycle arrest and apoptosis in GC cells (86). miR-99a expression in GC tissues was inversely correlated with IGFR1 expression and suppressed invasion and migration in GC cells (131).
Conversely, miR-99b overexpression has previously been reported in H. Pylori positive intestinal GC (132). Yang et al. suggested a role of miR-99b in H. Pylori-induced gastric carcinogenesis after finding higher expression of miR-99b in the H. Pylori positive GC samples compared to the negative samples, as well as in the H. Pylori infected GC cells (133). miR-99b promoted autophagy by targeting mTOR and thus negatively regulated proliferation of GC cells (133).
A recent report also showed that miR-99a and miR-491 were associated with resistance to CDDP in vitro (87). Upregulation of both were found in CDDP resistant GC cells as Calpain small subunit 1 (CAPNS1) was downregulated. Inhibition of miR-99a and miR-491 or overexpression of CAPNS1 foster CDDP sensitivity on resistant GC cells like the opposite increased CDDP resistance (87).
Zhang et al. observed that GC patients with high expression level of miR-145-3p, miR-125b-5p and miR-99a-5p had a poorer prognosis than those with a low expression level of miR-145-3p, miR-125b-5p and miR-99a-5p (134).
miR-498
A thought-provoking study previously reported on the actions of vitamin D in ovarian cancer cells, showed that vitamin D induced the expression of miR-498 through its binding to the VDRE located in the 5 ‘regulatory region of the miR-498 gene and directly targeted the 3'UTR of hTERT (human telomerase reverse transcriptase) mRNA (135). hTERT downregulation resulted in cell death and growth suppression not only in ovarian cancer cell lines, but also in other cancer cell lines, suggesting that this hTERT downregulation by vitamin D through
miR-498 might be found in other vitamin D sensitive cancer (135).
In two previous publications, miR-498 downregulation in GC cells and tissues has been reported (88,89) and associated with poorer prognosis in GC patients (89).
In GC, miR-498 overexpression inhibited invasion, proliferation and overcome CDDP resistance, in vitro and in vivo (88). Also, miR-498 inhibited cell proliferation and metastasis in GC cells through blocking EMT(Epithelial to mesenchymal transition) and AKT pathways (89). The data from these two studies showed that BMI-1 (B lymphoma Mo-MLV insertion region 1 homolog) is overexpressed in GC (89) and a direct target of miR-498 (88,89). Furthermore, BMI-1 overexpression reversed the inhibiting functions of miR-498 in GC cells (88). Previously, BMI-1 has been suggested to play a role in hTERT-induced EMT and cell immortalization (136). Therefore, miR-498 could play a role as a tumor suppressor in GC.
miR-22
Vitamin D induced miR-22 expression in colon cancer cells in a VDR-dependent manner. The actions of vitamin D on cell migration were mediated by miR-22, as well as the down-regulation of known vitamin D target genes in human colon cancer cells. In vitro, the application of anti-miR-22 reduced the antiproliferative effects of vitamin D (137).
miR-22 was found downregulated in GC tissues and GC cells compared to normal controls (90,91,138) and is associated with more aggressive phenotype and worse survival (91). In vitro, miR-22 suppressed cell growth, migration and invasion in GC cells and metalloproteinase 14 and Snail were its direct targets. In vivo, miR-22 abrogated tumor growth and metastatic dissemination (91).
Also, miR-22 has been linked to the regulation of cell growth and motility through targeting Sp1 (Specificity protein 1 transcription factor) and CD151 (90,138). Transfection of miR-22 expression plasmid inhibited cell migration and invasion in GC cells. Sp1 was directly target of miR-22 and their expression levels in GC tissues were inversely correlated, thus playing a role as a tumor suppressor (90).
Another target of miR-22 is NLRP3 (NLR Family pyrin domain containing 3) that has been found overexpressed in GC. NLRP3 estimulates inflammasome activation and promotes gastric epithelial cell proliferation. Notably, H. Pylori infection prevents miR-22 expression and enhances NLRP3 expression, which could play an important role in GC tumorigenesis (139).
Moreover, miR-22 has been implicated in the development of CDDP chemoresistance in GC cells due to the negative regulation it exerts on the expression of ENO1 (Glycolytic enzyme enolase 1), which is overexpressed in GC and associated with poor prognosis and CDDP resistance (92). Restoration of miR-22 could be a potential therapeutic target or a chemoresistance marker.
miR-181 family
In humans, the miR-181 family consists of four distinct 5p mature forms: miR-181a-5p, miR-181b-5p, miR-181c-5p and miR-181d-5p. Their precursors also yield 3p mature forms, in lower levels. All 5p forms share the same seed region and miR-181 members can behave as oncogenes or tumor suppressor (140).
In cultured myeloid leukemia cells, vitamin D downregulated miR-181 family and increased expression of p27kip1 and p21cip1, induced monocytic proliferation and cell cycle arrest in G0/G1. Moreover, p27kip1 is a direct target of miR-181, thereby, the repression of miR-181 by vitamin D appears to mediate its effects on cell differentiation and cell cycle arrest (141). More evidence of Vitamin D repression effect on miR-181 expression comes from a study of chronic myeloid leukemia cells (142). Here, miR-181 downregulation was associated with an increased expression of Mcl-1 (Myeloid cell leukemia-1) a Bcl-2 family protein associated with relapse and survival in AML (Acute myeloid leukemia) and chemotherapy resistance (142).
The role of miR-181 as an oncogene in GC is supported by different studies.
In a recent study, miR-181a overexpression was found in GC tissues and cell lines and associated with worse overall survival and more advanced clinicopathological factors (larger tumor size, more lymph node and distant metastasis, and higher TNM stages). Caprin-1 was identified as a direct target of miR-181a (143). Both in vitro and in vivo, ectopic expression of miR-181a enhanced proliferation, migration, invasion and antiapoptotic effects (143).
In another study, although miR-181a was found overexpressed in GC tissues, no association with clinicopathological characteristics was demonstrated (144). Instead, in GC cells miR-181a stimulated cell proliferation, G1/S phase transition and suppression of apoptosis, mainly through downregulation of RASSF1A, but also of Bax protein and upregulation of CDC25A, cyclin A2 and Bcl-2 (144).
As well, high expression of miR-181b in GC tissues has been associated with worse five year overall survival in GC patients with stage II and III and its expression levels were significantly correlated to tumor size, pathological differentiation, depth of infiltration, pT, lymph node, pN, TNM stage and presence of distant metastasis (93). In another study, miR-181b overexpression was reported in GC tissues compared to normal gastric tissues and low expression levels were associated to better survival, even in patients with more advanced GC stages (94).
Conversely, a possible role as a tumor suppressor of miR-181a in GC has been suggested (95). The authors found that miR-181a was downregulated in GC tissues compared to normal samples and that lower levels of miR-181a were associated with pTNM stage. In vitro, miR-181a expression blocked GC cell proliferation, migration, and invasion by direct targeting of Prox1 (95).
In this line, miR-181b was observed significantly decreased in GC tissues (145). miR-181b overexpression in GC cells blocked cell viability, proliferation and reduced glucose consumption, lactate production and ATP concentration, suggesting that miR-181b may regulate the switching between glycolisis and oxidative mitochoncrial metabolism for ATP production, by direct targeting of Hexokinase 2 (HK2), an enzyme involved in glycolysis (145).
miR-106b
Treatment with vitamin D, in vivo, lead to an increased expression of miR-106b in the prostate tissue and contributes to the regulation of p21(waf/cip) (146), a cell cycle inhibitor.
In GC, miR-106b is overexpressed in tumor tissues and cancer cells compared to non-tumorous and is suggested as a marker for diagnosis (96,147-149). Besides, miR-106b was found at a significantly higher level in the plasma of GC patients than in controls and was reduced in postoperative samples compared to preoperative (150). miR-106b belongs to a highly conserved cluster, form by miR-106b, miR-93 and miR-25, also upregulated in GC (151).
Overexpression of miR-106b in GC downregulates p21 (152,153). In vitro, miR-106b expression levels in GC cells increased according to the degree of malignancy and this upregulation shortened the G0/G1 phase and reduced the expression levels of p21 and E2F5, with no effects on migration and invasion (154).
Downregulation of miR-106b promoted apoptosis of GC cells via inhibiting JAK1/STAT3 signaling pathway, in vitro and in vivo (149). Zhu et al. showed that ALEX1, a member of the armadillo family, was a direct target of miR-106 in GC cells and its up-regulation restored cellular apoptosis induced by the miR-106b inhibitor (149).
Moreover, miR-106b is upregulated in cancer associated fibroblasts compared to normal fibroblats in GC and patients with high miR-106b stromal expression were associated with shorter overall survival (97). Its oncogenic effect was linked to an increase in migration and invasion, by targeting PTEN (97).
miR-532-3p
Recently, it has been explored the effect of vitamin D supplementation versus placebo in healthy subjects on plasma miRNA profile, but no consistent findings were reported, except a significant correlation between serum vitamin D and miR-532-3p expression at baseline and a significant change in miR-221 expression from baseline to 12 months but in placebo group (155).
RAB3A-interacting protein (Rab3IP) is a Rab-specific GEF (Guanine nucleotide exchange factor) and a major activator of Rab proteins. Rab3IP is overexpressed in GC cells and tissues and interacts with SSX2, inducing an aggressive phenotype of GC cells and its expression is correlated with markers involved in the EMT (156). Guo et al. reported that miR-532-3p was downregulated in GC samples compared to normal gastric tissues and that RAB3IP is a direct target of miR-532-3p in GC (98). As well, RAB3IP transcript and protein expression levels were higher in GC tissues than in normal gastric mucosa samples (98). Furthermore, expression level of Rab3IP was associated with tumor size, T stage, differentiation, N-stage of lymph nodes, and serum tumor marker levels CEA and CA-724 (98).
In contrast, Dai et al. found that miR-532-3p was overexpressed in GC tissues compared to non-cancerous tissues and might be involved in regulation of APC/B-catenin signaling (99).
Conclusions
We think that there is convincing evidence that vitamin D deficiency plays an important role in the appearance of molecular events that promote gastric carcinogenesis. Also, here we have reviewed that, in vitro and in vivo, vitamin D could modulate the expression of some miRNAs that are deregulated in GC tissue with respect to normal and in GC cells. Furthermore, vitamin D and some miRNAs might be capable of modulating the appearance of chemoresistance at the cellular level.
In contrast, vitamin D supplementation studies have not shown a reduction in the risk of GC either in the general population or in relapse in patients with GC operated on, but it is possible that the appropriate variables have not been chosen to demonstrate its benefit.
The low toxicity profile of vitamin D and its analogues, the ubiquity in the body of the VDR, as well as the important role that miRNAs have in protein expression regulation and possible synergies with vitamin D, project new working hypotheses that deserve to be explored.
Acknowledgments
Funding: We want to thank
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Alfons Navarro, Joan Josep Castellano and Marina Díaz-Beyá) for the series “Clinic and Therapeutic Potential of Non-coding RNAs in Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure forms (available at https://dx.doi.org/10.21037/tcr-20-2813). The series “Clinic and Therapeutic Potential of Non-coding RNAs in Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther 2014;40:250-60. [Crossref] [PubMed]
- Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J 2014;55:621-8. [Crossref] [PubMed]
- Park B, Shin A, Park SK, et al. Ecological study for refrigerator use, salt, vegetable, and fruit intakes, and gastric cancer. Cancer Causes Control 2011;22:1497-502. [Crossref] [PubMed]
- Choi IJ, Kim CG, Lee JY, et al. Family History of Gastric Cancer and Helicobacter pylori Treatment. N Engl J Med 2020;382:427-36. [Crossref] [PubMed]
- Correa P. Gastric cancer: two epidemics? Dig Dis Sci 2011;56:1585-6; author reply 1586. [Crossref] [PubMed]
- Du C, Yang S, Zhao X, et al. Pathogenic roles of alterations in vitamin D and vitamin D receptor in gastric tumorigenesis. Oncotarget 2017;8:29474-86. [Crossref] [PubMed]
- Milano AF. 20-Year Comparative Survival and Mortality of Cancer of the Stomach by Age, Sex, Race, Stage, Grade, Cohort Entry Time-Period, Disease Duration & Selected ICD-O-3 Oncologic Phenotypes: A Systematic Review of 157,258 Cases for Diagnosis Years 1973-2014: (SEER*Stat 8.3.4). J Insur Med 2019;48:5-23. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Muro K, Lordick F, Tsushima T, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 2019;30:34-43. [Crossref] [PubMed]
- Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Zhou M-L, Kang M, Li G-C, et al. Postoperative chemoradiotherapy versus chemotherapy for R0 resected gastric cancer with D2 lymph node dissection: an up-to-date meta-analysis. World J Surg Oncol 2016;14:209. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Noh SH, Park SR, Yang H-K, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389-96. [Crossref] [PubMed]
- Han D-S, Suh Y-S, Kong S-H, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012;30:3834-40. [Crossref] [PubMed]
- Han D-S, Suh Y-S, Kong S-H, et al. Outcomes of surgery aiming at curative resection in good responder to induction chemotherapy for gastric cancer with distant metastases. J Surg Oncol 2013;107:511-6. [Crossref] [PubMed]
- Charalampakis N, Economopoulou P, Kotsantis I, et al. Medical management of gastric cancer: a 2017 update. Cancer Med 2018;7:123-33. [Crossref] [PubMed]
- Baek D, Villén J, Shin C, et al. The impact of microRNAs on protein output. Nature 2008;455:64-71. [Crossref] [PubMed]
- Selbach M, Schwanhäusser B, Thierfelder N, et al. Widespread changes in protein synthesis induced by microRNAs. Nature 2008;455:58-63. [Crossref] [PubMed]
- Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005;433:769-73. [Crossref] [PubMed]
- Ma Y, Trump DL, Johnson CS. Vitamin D and miRNAs in cancer. Curr Gene Ther 2014;14:269-75. [Crossref] [PubMed]
- Zeljic K, Supic G, Magic Z. New insights into vitamin D anticancer properties: focus on miRNA modulation. Mol Genet Genomics 2017;292:511-24. [Crossref] [PubMed]
- Marano L, D’Ignazio A, Cammillini F, et al. Comparison between 7th and 8th edition of AJCC TNM staging system for gastric cancer: old problems and new perspectives. Transl Gastroenterol Hepatol 2019;4:22
- Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol 2003;21:3647-50. [Crossref] [PubMed]
- Wang W, Sun Z, Deng JY, et al. A novel nomogram individually predicting disease-specific survival after D2 gastrectomy for advanced gastric cancer. Cancer Commun (Lond) 2018;38:23. [Crossref] [PubMed]
- van den Ende T, Ter Veer E, Mali RMA, et al. Prognostic and Predictive Factors for the Curative Treatment of Esophageal and Gastric Cancer in Randomized Controlled Trials: A Systematic Review and Meta-Analysis. Cancers 2019;11:530. [Crossref] [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [Crossref] [PubMed]
- Tan IB, Ivanova T, Lim KH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology 2011;141:476-85, 485.e1-11.
- Shah MA, Khanin R, Tang L, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res 2011;17:2693-701. [Crossref] [PubMed]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735-40. [PubMed]
- Petrelli F, Berenato R, Turati L, et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2017;8:148-63. [Crossref] [PubMed]
- Jiménez Fonseca P, Carmona-Bayonas A, Hernández R, et al. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: real-world data from the AGAMENON National Cancer Registry. Br J Cancer 2017;117:775-82. [Crossref] [PubMed]
- Yepes S, López R, Andrade RE, et al. Co-expressed miRNAs in gastric adenocarcinoma. Genomics 2016;108:93-101. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Sohn BH, Hwang J-E, Jang H-J, et al. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clin Cancer Res 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Tirino G, Pompella L, Petrillo A, et al. What’s New in Gastric Cancer: The Therapeutic Implications of Molecular Classifications and Future Perspectives. Int J Mol Sci 2018;19:2659. [Crossref] [PubMed]
- Li Y, Bai W, Zhang X. Identifying heterogeneous subtypes of gastric cancer and subtype-specific subpaths of microRNA-target pathways. Mol Med Rep 2018;17:3583-90. [PubMed]
- Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726-76. [Crossref] [PubMed]
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007;7:684-700. [Crossref] [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [Crossref] [PubMed]
- Campbell MJ. Vitamin D and the RNA transcriptome: more than mRNA regulation. Front Physiol 2014;5:181. [Crossref] [PubMed]
- Shi Q, Han XP, Yu J, et al. Decreased vitamin D receptor protein expression is associated with progression and poor prognosis of colorectal cancer patients. Int J Clin Exp Pathol 2020;13:746-55. [PubMed]
- Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc 2008;67:115-27. [Crossref] [PubMed]
- Grant WB, Moukayed M. Vitamin D3 from Ultraviolet-B Exposure or Oral Intake in Relation to Cancer Incidence and Mortality. Curr Nutr Rep 2019;8:203-11. [Crossref] [PubMed]
- Bandera Merchan B, Morcillo S, Martin-Nuñez G, et al. The role of vitamin D and VDR in carcinogenesis: Through epidemiology and basic sciences. J Steroid Biochem Mol Biol 2017;167:203-18. [Crossref] [PubMed]
- Camara AB, Brandao IA. The Role of Vitamin D and Sunlight Incidence in Cancer. Anticancer Agents Med Chem 2019;19:1418-36. [Crossref] [PubMed]
- Scragg R, Khaw KT, Toop L, et al. Monthly High-Dose Vitamin D Supplementation and Cancer Risk: A Post Hoc Analysis of the Vitamin D Assessment Randomized Clinical Trial. JAMA Oncol 2018;4:e182178. [Crossref] [PubMed]
- Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684-96. [Crossref] [PubMed]
- Manson JE, Cook NR, Lee I-M, et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 2019;380:33-44. [Crossref] [PubMed]
- Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev 2014;CD007469. [Crossref] [PubMed]
- Keum N, Lee DH, Greenwood DC, et al. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol 2019;30:733-43. [Crossref] [PubMed]
- Khayatzadeh S, Feizi A, Saneei P, et al. Vitamin D intake, serum Vitamin D levels, and risk of gastric cancer: A systematic review and meta-analysis. J Res Med Sci 2015;20:790-6. [Crossref] [PubMed]
- Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 2012;308:1898-905. [Crossref] [PubMed]
- Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180-8. [Crossref] [PubMed]
- Urashima M, Ohdaira H, Akutsu T, et al. Effect of Vitamin D Supplementation on Relapse-Free Survival Among Patients With Digestive Tract Cancers: The AMATERASU Randomized Clinical Trial. JAMA 2019;321:1361-9. [Crossref] [PubMed]
- Urashima M, Okuyama M, Akutsu T, et al. Effect of Vitamin D Supplementation on Survival of Digestive Tract Cancer Patients with Low Bioavailable 25-Hydroxyvitamin D levels: A Post Hoc Analysis of the AMATERASU Randomized Clinical Trial. Cancers 2020;12:347. [Crossref] [PubMed]
- Vyas N, Companioni RC, Tiba M, et al. Association between serum vitamin D levels and gastric cancer: A retrospective chart analysis. World J Gastrointest Oncol 2016;8:688-94. [Crossref] [PubMed]
- Durak Ş, Gheybi A, Demirkol Ş, et al. The effects of serum levels, and alterations in the genes of binding protein and receptor of vitamin D on gastric cancer. Mol Biol Rep 2019;46:6413-20. [Crossref] [PubMed]
- Ren C, Qiu M, Wang D, et al. Prognostic effects of 25-hydroxyvitamin D levels in gastric cancer. J Transl Med 2012;10:16. [Crossref] [PubMed]
- Bao A, Li Y, Tong Y, et al. Tumor-suppressive effects of 1, 25-dihydroxyvitamin D3 in gastric cancer cells. Hepatogastroenterology 2013;60:943-8. [PubMed]
- Bao A, Li Y, Tong Y, et al. 1,25-Dihydroxyvitamin D3 and cisplatin synergistically induce apoptosis and cell cycle arrest in gastric cancer cells. Int J Mol Med 2014;33:1177-84. [Crossref] [PubMed]
- Mahendra A. Vitamin D and gastrointestinal cancer. J Lab Physicians 2018;10:1-5. [Crossref] [PubMed]
- Han C, Ni Z, Yuan T, et al. Influence of serum vitamin D level on Helicobacter pylori eradication: A multi-center, observational, prospective and cohort study. J Dig Dis 2019;20:421-6. [Crossref] [PubMed]
- Sohel MH. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev Life Sci 2016;10:175-86. [Crossref]
- Bartel DP. Metazoan MicroRNAs. Cell 2018;173:20-51. [Crossref] [PubMed]
- Yang X, Du WW, Li H, et al. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res 2013;41:9688-704. [Crossref] [PubMed]
- Shrestha S, Hsu S-D, Huang W-Y, et al. A systematic review of microRNA expression profiling studies in human gastric cancer. Cancer Med 2014;3:878-88. [Crossref] [PubMed]
- Yan W, Qian L, Chen J, et al. Comparison of Prognostic MicroRNA Biomarkers in Blood and Tissues for Gastric Cancer. J Cancer 2016;7:95-106. [Crossref] [PubMed]
- Asadi M, Shanehbandi D, Zafari V, et al. Transcript Level of MicroRNA Processing Elements in Gastric Cancer. J Gastrointest Cancer 2019;50:855-9. [Crossref] [PubMed]
- Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 2004;101:2999-3004. [Crossref] [PubMed]
- Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 2009;27:5848-56. [Crossref] [PubMed]
- Tsai MM, Wang CS, Tsai CY, et al. Potential Diagnostic, Prognostic and Therapeutic Targets of MicroRNAs in Human Gastric Cancer. Int J Mol Sci 2016;17:945. [Crossref] [PubMed]
- Janssen HLA, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685-94. [Crossref] [PubMed]
- miRagen Therapeutics, Inc. A Phase 1 Dose-ranging Study to Investigate the Safety, Tolerability, and Pharmacokinetics of MRG-106 Following Local Intratumoral, Subcutaneous, and Intravenous Administration in Subjects With Various Lymphomas and Leukemias [Internet]. clinicaltrials.gov; 2020 Feb [cited 2020 Aug 10]. Report No.: NCT02580552. Available online: https://clinicaltrials.gov/ct2/show/NCT02580552
- Ahadi A. Dysregulation of miRNAs as a signature for diagnosis and prognosis of gastric cancer and their involvement in the mechanism underlying gastric carcinogenesis and progression. IUBMB Life 2020;72:884-98. [Crossref] [PubMed]
- Negri M, Gentile A, de Angelis C, et al. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients 2020;12:1798. [Crossref] [PubMed]
- Fassan M, Pizzi M, Realdon S, et al. The HER2-miR125a5p/miR125b loop in gastric and esophageal carcinogenesis. Hum Pathol 2013;44:1804-10. [Crossref] [PubMed]
- Wu S, Liu F, Xie L, et al. miR-125b Suppresses Proliferation and Invasion by Targeting MCL1 in Gastric Cancer. BioMed Res Int 2015;2015:365273. [Crossref] [PubMed]
- Zhang X, Yao J, Guo K, et al. The functional mechanism of miR-125b in gastric cancer and its effect on the chemosensitivity of cisplatin. Oncotarget 2017;9:2105-19. [Crossref] [PubMed]
- Sui M, Jiao A, Zhai H, et al. Upregulation of miR-125b is associated with poor prognosis and trastuzumab resistance in HER2-positive gastric cancer. Exp Ther Med 2017;14:657-63. [Crossref] [PubMed]
- Zeng JF, Ma XQ, Wang LP, et al. MicroRNA-145 exerts tumor-suppressive and chemo-resistance lowering effects by targeting CD44 in gastric cancer. World J Gastroenterol 2017;23:2337-45. [Crossref] [PubMed]
- Jiang SB, He XJ, Xia YJ, et al. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. Onco Targets Ther 2016;9:2305-15. [PubMed]
- Wang Z, Zhao Z, Yang Y, et al. MiR-99b-5p and miR-203a-3p Function as Tumor Suppressors by Targeting IGF-1R in Gastric Cancer. Sci Rep 2018;8:10119. [Crossref] [PubMed]
- Zhang Y, Xu W, Ni P, et al. MiR-99a and MiR-491 Regulate Cisplatin Resistance in Human Gastric Cancer Cells by Targeting CAPNS1. Int J Biol Sci 2016;12:1437-47. [Crossref] [PubMed]
- Zhao T, Chen Y, Sheng S, et al. Upregulating microRNA-498 inhibits gastric cancer proliferation invasion and chemoresistance through inverse interaction of Bmi1. Cancer Gene Ther 2019;26:366-73. [Crossref] [PubMed]
- You D, Wang D, Liu P, et al. MicroRNA-498 inhibits the proliferation, migration and invasion of gastric cancer through targeting BMI-1 and suppressing AKT pathway. Hum Cell 2020;33:366-76. [Crossref] [PubMed]
- Guo M-M, Hu L-H, Wang Y-Q, et al. miR-22 is down-regulated in gastric cancer, and its overexpression inhibits cell migration and invasion via targeting transcription factor Sp1. Med Oncol 2013;30:542. [Crossref] [PubMed]
- Zuo QF, Cao LY, Yu T, et al. MicroRNA-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and Snail. Cell Death Dis 2015;6:e2000. [Crossref] [PubMed]
- Qian X, Xu W, Xu J, et al. Enolase 1 stimulates glycolysis to promote chemoresistance in gastric cancer. Oncotarget 2017;8:47691-708. [Crossref] [PubMed]
- Gao Y, Xu Z, Yuan F, et al. Correlation of Expression Levels of Micro Ribonucleic Ccid-10b (miR-10b) and Micro Ribonucleic Acid-181b (miR-181b) with Gastric Cancer and Its Diagnostic Significance. Med Sci Monit 2018;24:7988-95. [Crossref] [PubMed]
- Jiang J, Zheng X, Xu X, et al. Prognostic Significance of miR-181b and miR-21 in Gastric Cancer Patients Treated with S-1/Oxaliplatin or Doxifluridine/Oxaliplatin. PLoS One 2011;6:e23271. [Crossref] [PubMed]
- Lin F, Li Y, Yan S, et al. MicroRNA-181a inhibits tumor proliferation, invasiveness, and metastasis and is downregulated in gastric cancer. Oncol Res 2015;22:75-84. [Crossref] [PubMed]
- Arias Sosa LA, Cuspoca Orduz AF, Bernal Gómez BM. Deregulation of microRNAs in gastric cancer: up regulation by miR-21 and miR-106. Rev Gastroenterol Peru 2017;37:65-70. [PubMed]
- Yang TS, Yang XH, Chen X, et al. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett 2014;588:2162-9. [Crossref] [PubMed]
- Guo W, Chen Z, Chen Z, et al. Promotion of Cell Proliferation through Inhibition of Cell Autophagy Signalling Pathway by Rab3IP is Restrained by MicroRNA-532-3p in Gastric Cancer. J Cancer 2018;9:4363-73. [Crossref] [PubMed]
- Dai X, Liu J, Guo X, et al. Circular RNA circFGD4 suppresses gastric cancer progression via modulating miR-532-3p/APC/β-catenin signalling pathway. Clin Sci (Lond Engl 1979) 2020;134:1821-39.
- Banzhaf-Strathmann J, Edbauer D. Good guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell Commun Signal 2014;12:30. [Crossref] [PubMed]
- Mohri T, Nakajima M, Takagi S, et al. MicroRNA regulates human vitamin D receptor. Int J Cancer 2009;125:1328-33. [Crossref] [PubMed]
- Komagata S, Nakajima M, Takagi S, et al. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol 2009;76:702-9. [Crossref] [PubMed]
- Huss L, Butt ST, Borgquist S, et al. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res 2019;21:84. [Crossref] [PubMed]
- McCain S, Trainor J, McManus DT, et al. Vitamin D receptor as a marker of prognosis in oesophageal adenocarcinoma: a prospective cohort study. Oncotarget 2018;9:34347-56. [Crossref] [PubMed]
- Wen Y, Da M, Zhang Y, et al. Alterations in vitamin D signaling pathway in gastric cancer progression: a study of vitamin D receptor expression in human normal, premalignant, and malignant gastric tissue. Int J Clin Exp Pathol 2015;8:13176-84. [PubMed]
- Seubwai W, Wongkham C, Puapairoj A, et al. Overexpression of vitamin D receptor indicates a good prognosis for cholangiocarcinoma: implications for therapeutics. Cancer 2007;109:2497-505. [Crossref] [PubMed]
- Riquelme I, Tapia O, Leal P, et al. miR-101-2, miR-125b-2 and miR-451a act as potential tumor suppressors in gastric cancer through regulation of the PI3K/AKT/mTOR pathway. Cell Oncol Dordr 2016;39:23-33. [Crossref] [PubMed]
- Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Yang ZX, Lu CY, Yang YL, et al. MicroRNA-125b expression in gastric adenocarcinoma and its effect on the proliferation of gastric cancer cells. Mol Med Rep 2013;7:229-32. [Crossref] [PubMed]
- Wu JG, Wang JJ, Jiang X, et al. MiR-125b promotes cell migration and invasion by targeting PPP1CA-Rb signal pathways in gastric cancer, resulting in a poor prognosis. Gastric Cancer 2015;18:729-39. [Crossref] [PubMed]
- Chang S, He S, Qiu G, et al. MicroRNA-125b promotes invasion and metastasis of gastric cancer by targeting STARD13 and NEU1. Tumour Biol 2016;37:12141-51. [Crossref] [PubMed]
- Jing JC, Feng Z, Chen ZH, et al. KDM4B promotes gastric cancer metastasis by regulating miR-125b-mediated activation of Wnt signaling. J Cell Biochem. 2018; [Epub ahead of print]. [PubMed]
- Xu L, Zhang Y, Tang J, et al. The Prognostic Value and Regulatory Mechanisms of microRNA-145 in Various Tumors: A Systematic Review and Meta-analysis of 50 Studies. Cancer Epidemiol Biomarkers Prev 2019;28:867-81. [Crossref] [PubMed]
- Takagi T, Iio A, Nakagawa Y, et al. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology 2009;77:12-21. [Crossref] [PubMed]
- Chang S, Gao L, Yang Y, et al. miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Oncotarget 2015;6:7675-85. [Crossref] [PubMed]
- Wang J, Sun Z, Yan S, et al. Effect of miR-145 on gastric cancer cells. Mol Med Rep 2019;19:3403-10. [Crossref] [PubMed]
- Xu WX, Liu Z, Deng F, et al. MiR-145: a potential biomarker of cancer migration and invasion. Am J Transl Res 2019;11:6739-53. [PubMed]
- Gao P, Xing AY, Zhou GY, et al. The molecular mechanism of microRNA-145 to suppress invasion-metastasis cascade in gastric cancer. Oncogene 2013;32:491-501. [Crossref] [PubMed]
- Chang S, Gao Z, Yang Y, et al. miR-99b-3p is induced by vitamin D3 and contributes to its antiproliferative effects in gastric cancer cells by targeting HoxD3. Biol Chem. 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Hwang J, Min BH, Jang J, et al. MicroRNA Expression Profiles in Gastric Carcinogenesis. Sci Rep 2018;8:14393. [Crossref] [PubMed]
- Xing AY, Wang YW, Su ZX, et al. Catenin-δ1, negatively regulated by miR-145, promotes tumour aggressiveness in gastric cancer. J Pathol 2015;236:53-64. [Crossref] [PubMed]
- Chen JJ, Cai WY, Liu XW, et al. Reverse Correlation between MicroRNA-145 and FSCN1 Affecting Gastric Cancer Migration and Invasion. PLoS One 2015;10:e0126890. [Crossref] [PubMed]
- Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol 2011;224:289-300. [Crossref] [PubMed]
- Zheng L, Pu J, Qi T, et al. miRNA-145 targets v-ets erythroblastosis virus E26 oncogene homolog 1 to suppress the invasion, metastasis, and angiogenesis of gastric cancer cells. Mol Cancer Res 2013;11:182-93. [Crossref] [PubMed]
- Wang W, Dong LP, Zhang N, et al. Role of cancer stem cell marker CD44 in gastric cancer: a meta-analysis. Int J Clin Exp Med 2014;7:5059-66. [PubMed]
- Jiang Y, He Y, Li H, et al. Expressions of putative cancer stem cell markers ABCB1, ABCG2, and CD133 are correlated with the degree of differentiation of gastric cancer. Gastric Cancer 2012;15:440-50. [Crossref] [PubMed]
- Kaufhold S, Garbán H, Bonavida B. Yin Yang 1 is associated with cancer stem cell transcription factors (SOX2, OCT4, BMI1) and clinical implication. J Exp Clin Cancer Res 2016;35:84. [Crossref] [PubMed]
- Xu N, Papagiannakopoulos T, Pan G, et al. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 2009;137:647-58. [Crossref] [PubMed]
- Basati G, Mohammadpour H, Emami Razavi A. Association of High Expression Levels of SOX2, NANOG, and OCT4 in Gastric Cancer Tumor Tissues with Progression and Poor Prognosis. J Gastrointest Cancer 2020;51:41-7. [Crossref] [PubMed]
- Chen D, Chen Z, Jin Y, et al. MicroRNA-99 family members suppress Homeobox A1 expression in epithelial cells. PloS One 2013;8:e80625. [Crossref] [PubMed]
- Xu X-L, Guo A-X, Pan Q-Y, et al. MiR-99a suppresses cell migration and invasion by regulating IGF1R in gastric cancer. Eur Rev Med Pharmacol Sci 2019;23:7375-82. [PubMed]
- Chang H, Kim N, Park JH, et al. Different microRNA expression levels in gastric cancer depending on Helicobacter pylori infection. Gut Liver 2015;9:188-96. [Crossref] [PubMed]
- Yang L, Li C, Jia Y. MicroRNA-99b promotes Helicobacter pylori-induced autophagyand suppresses carcinogenesis by targeting mTOR. Oncol Lett 2018;16:5355-60. [Crossref] [PubMed]
- Zhang C, Zhang CD, Ma MH, et al. Three-microRNA signature identified by bioinformatics analysis predicts prognosis of gastric cancer patients. World J Gastroenterol 2018;24:1206-15. [Crossref] [PubMed]
- Kasiappan R, Shen Z, Tse AKW, et al. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J Biol Chem 2012;287:41297-309. [Crossref] [PubMed]
- Qiao B, Chen Z, Hu F, et al. BMI-1 activation is crucial in hTERT-induced epithelial-mesenchymal transition of oral epithelial cells. Exp Mol Pathol 2013;95:57-61. [Crossref] [PubMed]
- Alvarez-Díaz S, Valle N, Ferrer-Mayorga G, et al. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum Mol Genet 2012;21:2157-65. [Crossref] [PubMed]
- Wang X, Yu H, Lu X, et al. MiR-22 suppresses the proliferation and invasion of gastric cancer cells by inhibiting CD151. Biochem Biophys Res Commun 2014;445:175-9. [Crossref] [PubMed]
- Li S, Liang X, Ma L, et al. MiR-22 sustains NLRP3 expression and attenuates H. pylori-induced gastric carcinogenesis. Oncogene 2018;37:884-96. [Crossref] [PubMed]
- Indrieri A, Carrella S, Carotenuto P, et al. The Pervasive Role of the miR-181 Family in Development, Neurodegeneration, and Cancer. Int J Mol Sci 2020;21:2092. [Crossref] [PubMed]
- Wang X, Gocek E, Liu C-G, et al. MicroRNAs181 regulate the expression of p27Kip1 in human myeloid leukemia cells induced to differentiate by 1,25-dihydroxyvitamin D3. Cell Cycle 2009;8:736-41. [Crossref] [PubMed]
- Zimmerman EI, Dollins CM, Crawford M, et al. Lyn kinase-dependent regulation of miR181 and myeloid cell leukemia-1 expression: implications for drug resistance in myelogenous leukemia. Mol Pharmacol 2010;78:811-7. [Crossref] [PubMed]
- Lu Q, Chen Y, Sun D, et al. MicroRNA-181a Functions as an Oncogene in Gastric Cancer by Targeting Caprin-1. Front Pharmacol 2019;9:1565. [Crossref] [PubMed]
- Yu J, Qi J, Sun X, et al. MicroRNA-181a promotes cell proliferation and inhibits apoptosis in gastric cancer by targeting RASSF1A. Oncol Rep 2018;40:1959-70. [Crossref] [PubMed]
- Li L-Q, Yang Y, Chen H, et al. MicroRNA-181b inhibits glycolysis in gastric cancer cells via targeting hexokinase 2 gene. Cancer Biomark 2016;17:75-81. [Crossref] [PubMed]
- Thorne JL, Maguire O, Doig CL, et al. Epigenetic control of a VDR-governed feed-forward loop that regulates p21(waf1/cip1) expression and function in non-malignant prostate cells. Nucleic Acids Res 2011;39:2045-56. [Crossref] [PubMed]
- Peng Q, Shen Y, Lin K, et al. Comprehensive and integrative analysis identifies microRNA-106 as a novel non-invasive biomarker for detection of gastric cancer. J Transl Med 2018;16:127. [Crossref] [PubMed]
- Guo J, Miao Y, Xiao B, et al. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol 2009;24:652-7. [Crossref] [PubMed]
- Zhu Z, Yang Q, Zhang B, et al. miR-106b Promotes Metastasis of Early Gastric Cancer by Targeting ALEX1 in Vitro and in Vivo. Cell Physiol Biochem 2019;52:606-16. [Crossref] [PubMed]
- Tsujiura M, Ichikawa D, Komatsu S, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer 2010;102:1174-9. [Crossref] [PubMed]
- Zhang R, Wang W, Li F, et al. MicroRNA-106b~25 expressions in tumor tissues and plasma of patients with gastric cancers. Med Oncol 2014;31:243. [Crossref] [PubMed]
- Kim Y-K, Yu J, Han TS, et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res 2009;37:1672-81. [Crossref] [PubMed]
- Li F, Liu J, Li S. MicroRNA 106b ~ 25 cluster and gastric cancer. Surg Oncol 2013;22:e7-10. [Crossref] [PubMed]
- Yao YL, Wu XY, Wu JH, et al. Effects of microRNA-106 on proliferation of gastric cancer cell through regulating p21 and E2F5. Asian Pac J Cancer Prev APJCP 2013;14:2839-43. [Crossref] [PubMed]
- Jorde R, Svartberg J, Joakimsen RM, et al. Plasma profile of microRNA after supplementation with high doses of vitamin D3 for 12 months. BMC Res Notes 2012;5:245. [Crossref] [PubMed]
- Ren H, Xu Z, Guo W, et al. Rab3IP interacts with SSX2 and enhances the invasiveness of gastric cancer cells. Biochem Biophys Res Commun 2018;503:2563-8. [Crossref] [PubMed]