WSB2 as a target of Hedgehog signaling promoted the malignant biological behavior of Xuanwei lung cancer through regulating Wnt/β-catenin signaling
Introduction
Lung cancer represents the most leading cancer-related deaths in China and is increasing fast in recent year, especially in Xuanwei which is a county located in the northeast of Yunnan Province, China (1). The crude mortality rates of lung cancers in Xuanwei are usually higher than in other areas of China (2). Besides, Xuanwei lung cancer show some different characteristics, for example, the major type of lung cancer in Xuanwei is adenocarcinoma, and the incidence is remarkable higher in non-smoking women, in addition, the incidence peaks of lung cancer in Xuanwei, aged between 41 and 50, are younger than in other areas of the country (3). Despite some advent in the early diagnosis and treatment of Xuanwei lung cancer have been made in recent years, the over 5-year survival time for those patients still poor (4). Therefore, it is necessary to find effective bio-markers for lung cancer in Xuanwei.
WD repeat and suppressor of cytokine signaling (SOCS) box containing proteins (WSB) are the member of SOCS family. Up to date, only two sub-types WSB proteins have been identified, named as WSB1 and WSB2 (5). There were many researches which studied the relationship between WSB1 and cancer. For example, a study found that increased levels of WSB1 in primary osteosarcomas correlated with pulmonary metastatic potential (6). Besides, WSB1 also play a role in neuroblastoma cell growth and tumor progression (7). However, there was not much study on the function of WSB2 in cancer. Existing studies demonstrated that WSB1 and WSB2 contain eight WD-40 repeats and a SOCS box domain and show the similar levels (approximately 65%) and distribution. Thus, WSB1 and WSB2 potentially share some functional homology (8). Based on participation of WSB1 in the progression of cancer, and there have been no reports about the effect of the WSB2 on the development of Xuanwei lung cancer, therefore the functional role of WSB2 in Xuanwei lung cancer was explored here.
Accumulating evidence indicated that abnormal activation of Wnt/β-catenin pathway was correlated to carcinogenesis through regulating cell apoptosis, cell cycle progression and epithelial-mesenchymal transition (EMT) (9). For example, triptolide was found to trigger apoptotic cell death in osteosarcoma cells by blocking Wnt/β-catenin pathway (10). Besides, β4-subunit-mediated inactivation of Wnt/β-catenin signaling inhibited cell growth and cell cycle progression of hepatoma (11). In addition, down-regulated MAC30 expression inhibited cell invasion and EMT of breast cancer through suppressing Wnt/β-catenin signaling pathway (12). Importantly, a recent study revealed that WSB2 regulated the expression of β-catenin in melanoma cells (13). However, there was no report indicated that WSB2 regulated the development of Xuanwei lung cancer via Wnt/β-catenin signaling pathway.
Current study was designed to measure the expression of WSB2 in Xuanwei lung cancer tissues and cell lines. Furthermore, the function of WSB2 effect on the cell proliferation, apoptosis, cycle progression, EMT and invasion ability of Xuanwei lung cancer in vitro was studied. Besides, a xenograft tumor model was constructed to confirm the effect of WSB2 in vivo. In addition, the relationship between WSB2 and Wnt/β-catenin signaling pathway, and their function in Xuanwei lung cancer were explored. Overall, this study aims to provide a promising target for Xuanwei lung cancer.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2450).
Methods
Tissue samples
Human normal lung tissues (n=70), lung cancer tissues (n=68) and Xuanwei lung cancer tissues (n=66) (patients born in Xuanwei and lived in Xuanwei for more than 15 years) were collected from patients who did not receive radiochemotherapy before surgery and signed the informed consents. The tissues were used to measure the expression of WSB2, and the low and high expression in WSB2 was defined by the median of the WSB2 expression in 66 patients with Xuanwei lung cancer.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Yunnan Cancer Hospital (NO.: KY2019.57) and informed consent was taken from all the patients.
Cell culture and transfection
Human normal bronchial epithelial cell line BEAS-2B and lung cancer cell line A549 were acquired from Shanghai Kanglang Biotechnology Co., Ltd. (Shanghai, China). Xuanwei lung cancer cell line XWLC-05 was obtained from Yunnan Cancer Institute, China. All cells were maintained with Dulbecco’s Modified Eagle Medium (#11965092, Sigma-Aldrich, St. Louis, MO, USA)) containing 5% fetal bovine serum (#SH30070.02, HyClone, USA) and kept at 37 °C in 5% CO2. pcDNA3.1-WSB2 overexpression vector (OV-WSB2) and short hairpin RNA targeting WSB2 (sh-WSB2) which were synthesized by Genechem Co., Ltd. (Shanghai, China) were transfected into A549 and XWLC-05. In this paper, the negative control (NC) group was the cells without any treatment.
Quantitative reverse transcription-PCR (qRT-PCR)
The relative mRNA levels of WSB2 in tissues and cells were detected using qRT-PCR. Total RNA in Xuanwei lung cancer cells and tissues were isolated using TRIzol reagent (#DXT-15596018, Gibco, USA). After transcribed the extracted RNA into cDNA by using a Reverse Transcription Kit (PE013-01, AIVD, Guangdong, China), the qRT-PCR analysis was carried out with the ABI7300 system by using a SYBR green PCR kit (218073, Qiagen, Germany). Reaction system was as follows: cDNA 2.5 µL, primer (10 µmol/L) 2 µL, 2× Tag PCR Master Mix 12.5 µL, DEPC 8 µL, the total volume was 25 µL. Reaction was carried out at pre-denaturation at 95 °C for 2 min, denaturation at 95 °C for 15 s, annealing at 60 °C for 10 s, extending at 75 °C for 40 s, and finally extending at 75 °C for 2 min. U6 was selected as an internal reference. WSB2 level was calculated by using 2−ΔΔCt method.
Western blotting
The protein levels of WSB2, E-cadherin, N-cadherin, vimentin, c-Myc and β-catenin in cells were determined by Western blotting. A RIPA lysis buffer (orb348557, Biorbyt, UK) was used to extract total proteins from transfected A549 and XWLC-05. Ten percent sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, YB100915-12, Ybscience, Shanghai, China) was used to separate the extracted proteins, and then blotted onto polyvinylidene fluoride (PVDF) membranes (25900446, GE, USA). Then membrane was incubated with 5% skimmed milk at 25 °C for 1 h. After that, the primary antibodies against WSB2 (ab127176, Abc4am, USA), E-cadherin (PA512542, Pierce, USA), N-cadherin (PA529569, Pierce), vimentin (PA512321, Pierce), c-Myc (ab39688, Abcam), β-catenin (DXT-130-107-174, Miltenyi, USA) and GAPDH (PA515035, Pierce) were incubated at 4 °C overnight. All primary antibodies were diluted at the concentration of 1:1,000. Following that, secondary antibodies (20775, Millipore, USA) were incubated at 25 °C for 1 h. The enhanced chemiluminescence kit (orb90502, Biorbyt) was used to visualize the signals. GAPDH was the reference control. The optical density of blot was analyzed by using the ImageJ software.
3-(4,5-diethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-etrazolium, inner salt (MTS) assay
Cell viability was monitored by using MTS assay. Briefly, transfected A549 and XWLC-05 cells were plated into 96-well plates and maintained in an incubator at 37 °C with 5% CO2. Following 48 h, 20 µL of MTS (S12069, Yeyuan, Shanghai, China) was added and incubated for 2 h. Then the absorbance value at 490 nm was quantified using microplate autoreader (iMark, Bio-Rad, USA).
Flow cytometry
Cell apoptosis ratio and cell cycle distribution were analyzed using flow cytometry. Transfected A549 and XWLC-05 cells were collected and washed using cold phosphate buffer saline (PBS, DXT-103-070-525, Miltenyi, USA). After fixed the cells with absolute ethyl alcohol (A2877-1LAG, AG Scientific, Germany) at 4 °C for 24 h. Cells were stained with annexin V-FITC/propidium iodide (PI) staining according to manufacturer’s instruction. Then apoptotic cell population and cell cycle distribution were quantified using flow cytometry (CytoFLEX, Beckman Coulter, USA).
Transwell assay
The change of cell invasion ability was determined by using Transwell assay. Briefly, A549 and XWLC-05 cells transfection with WSB2 were plated in the upper chamber coated with Matrigel (356234, BD Biocoat, USA), while the lower chamber was added 500 µL DMEM medium containing 20% FBS and incubated for 8 h. Then, invaded cells in lower sides of Transwell were fixed with 95% ethanol (orb63597, Biorbyt) for 30 min. Following that, cells were incubated with 0.1% crystal violet (C8470, Solarbio, Shanghai, China) for 15 min and observed and photographed under microscope.
Animal experiments
Xenograft mouse model was used to examine tumor growth. The procedures for care and use of animals were approved by the Ethics Committee of the Third Affiliated Hospital of Kunming Medical University and all applicable institutional regulations and concerning the ethical use of animals were followed. In brief, transfected A549 and XWLC-05 cells (5×106) were incubated into the back of nude mice. Tumor volumes were measure twice a week and calculated according to the following equation: volume = (length×width2)/2. At 35 days, tumors were isolated from mice via anesthesia.
Statistical analyses
All data were presented as the mean ± standard deviation (SD). Statistical analysis was conducted by GraphPad Prism. T-tests, ANOVA and Chi-square tests were conducted to determine differences. P value less than 0.05 was considered statistically significant.
Results
WSB2 was frequently elevated in Xuanwei lung cancer tissues and cells
Several published literatures revealed that WSB was involved in the tumorigenesis and progression of various malignant tumors (5-8). Here, we firstly detected the levels of WSB2 in human normal lung tissue, lung cancer tissues and Xuanwei lung cancer tissues, and results revealed that WSB2 was aberrantly up-regulated in Xuanwei lung cancer and lung cancer tissue when compared with normal lung tissue. Interestingly, the expression level of WSB2 in Xuanwei lung cancer was significantly higher than that in lung cancer tissues (Figure 1A). Moreover, both mRNA (Figure 1B) and proteins levels (Figure 1C) of WSB2 in XWLC-05 cells were higher than in A549 cells. Above all the results indicated that there was a high expression of WSB2 in Xuanwei lung cancer tissues and cell lines. Clinical data analysis of the relationship between expression of WSB2 and clinical characteristics (Table 1), the results showed that WSB2 was negatively correlated with the TNM stage, lymph node metastasis and tumor size of Xuanwei lung cancer. While no obvious correlation between WSB2 and age, gender, smoking and differentiation was observed.
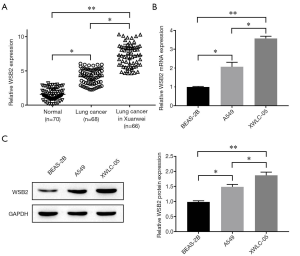
Table 1
| Clinical characteristics | Number of cases | Low expression of WSB2 (n=33) | High expression of WSB2 (n=33) | P |
|---|---|---|---|---|
| Age (years) | 0.7854 | |||
| >50 | 35 | 18 | 17 | |
| ≤50 | 31 | 15 | 16 | |
| Gender | 0.7451 | |||
| Male | 28 | 15 | 13 | |
| Female | 38 | 18 | 20 | |
| Smoking | 0.8031 | |||
| Yes | 41 | 20 | 21 | |
| No | 25 | 13 | 12 | |
| Differentiation | 0.7023 | |||
| Well/moderate | 44 | 20 | 24 | |
| Poor | 22 | 13 | 9 | |
| TNM stage | 0.0351 | |||
| I + II | 30 | 9 | 21 | |
| III + IV | 36 | 24 | 12 | |
| Tumor size | 0.0125 | |||
| >3 cm | 32 | 7 | 25 | |
| ≤3 cm | 34 | 26 | 8 | |
| Lymph node metastasis | 0.0184 | |||
| Negative | 29 | 21 | 8 | |
| Positive | 37 | 12 | 25 |
WSB2, WD repeat and SOCS box containing protein 2.
Knockdown of WSB2 suppressed the progression of Xuanwei lung cancer in vitro
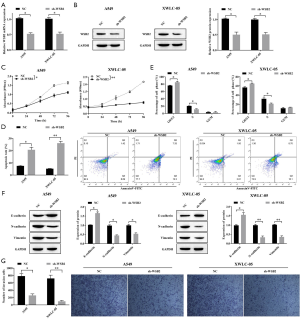
To further explore the effects of WSB2 on Xuanwei lung cancer, we manipulated the expression level of WSB2 in A549 and XWLC-05 by transfecting sh-WSB2. qRT-PCR (Figure 2A) and Western blotting (Figure 2B) results demonstrated WSB2 level was notably reduced in sh-WSB2 transfected cell, when compared with control. Moreover, The MTS results revealed that cell proliferation of A549 and XWLC-05 with WSB2 knockdown significantly decreased compared with control group (Figure 2C). Besides, flow cytometry analysis of cell apoptosis and cell cycle progression showed that knockdown of WSB2 in A549 and XWLC-05 promoted the cell apoptosis (Figure 2D) and effectively resulted in cell cycle blockage (Figure 2E). Moreover, knockdown of WSB2 suppressed EMT process in A549 and XWLC-05, as supported by the increase of E-cadherin and decrease of N-cadherin and vimentin in sh-WSB2 transfected cells (Figure 2F). Consistently, WSB2 knockdown also effectively reduced cell invasion of A549 and XWLC-05 (Figure 2G). Therefore, knockdown of WSB2 suppressed the progression of lung cancer in XWLC-05 more noticeable then A549.
Knockdown of WSB2 inhibited the tumor growth in vivo
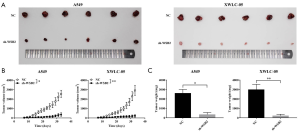
In addition, the xenograft model in nude mice was established by injecting the A549 and XWLC-05 which were knockdown of WSB2. Our data revealed WSB2 down-regulation effectively delayed tumor growth (Figure 3A,B,C). Hence, knockdown of WSB2 remarkably inhibited the tumor growth of lung cancer in Xuanwei.
WSB2 promoted the development of lung cancer in Xuanwei through regulated Wnt/β-catenin signaling
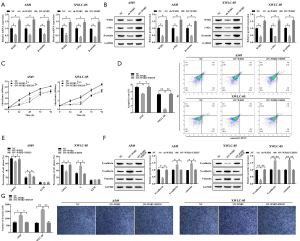
Increasing evidences demonstrated that Wnt/β-catenin signaling participate in the regulation of cancer (10-12). In this study, we also validated whether Wnt/β-catenin signaling was contributed to the tumor-promoting effect of WSB2. As anticipated, our results revealed WSB2 could modulate the expression levels of c-Myc and β-catenin, two key proteins in Wnt/β-catenin signaling. Compared with the NC group, the expression of c-Myc and β-catenin decreased in the sh-WSB2 group, but increased in the OV-WSB2 group (Figure 4A). Our data indicated that WSB2 regulated the Wnt/β-catenin signaling in lung cancer. Moreover, the weather WSB2 regulated the development of lung cancer through Wnt/β-catenin signaling was explored. The results showed that compared with the NC group, WSB2 up-regulation could enhance cell growth, cycle progression, EMT and invasion ability of A549 and XWLC-05, while inhibited the cell apoptosis. However, compared with the OV-WSB2 group, FH535 relieved the cancer promoting effect caused by overexpression of WSB2 (Figure 4B,C,D,E,F,G). In conclusion, WSB2 promoted the development of lung cancer, especially Xuanwei lung cancer, which could be by activating Wnt/β-catenin pathway.
Discussion
Since the mid-1970s, the morbidity and mortality of lung cancer in Xuanwei continuously rises, and ranks the first in China (14). Therefore, looking for an effective therapeutic target for targeted therapy is an important research direction (15). Our current findings indicated that WSB2 was frequently over-expressed in both Xuanwei lung cancer tissues and cells, and WSB2 act as oncogene in the tumorigenesis of Xuanwei lung cancer through regulating Wnt/β-catenin signaling.
Based on the severity of lung cancer in Xuanwei, increasingly researchers are paying attention to it. A recent study revealed that the predisposing risk factor for Xuanwei lung cancer was delayed effect of the indoor air pollution over 30 years (16). Mostly of the researches on lung cancer in Xuanwei studied the complex interaction between environmental factor or tobacco use and lung cancer risk (17). As one of the WSB proteins, there are plenty of researches focused on the function of WSB1, conversely, there have been surprisingly few studies of the function of WSB2. To our current knowledge, numerous researches have found the frequently expression of WSB1 in various types of cancer (18). Such as, WSB1 knockdown significantly reduced the invasiveness of hormone receptor negative breast cancer cells (19). Furthermore, there was a research indicated that miR-592 could inhibit aerobic glycolysis and growth hepatocellular carcinoma through directly targeting WSB1 (20). Besides, a study reported that WSB1 could rescue tumor cell senescence-induced by oncogene through promoting the ubiquitination degradation of ATM (21). While considering WSB2, one study found that WSB2 gene might be involved in the development of myeloid leukemia through binding to the COOH-terminal region of colony-stimulating factor receptor and subsequently affecting its level and function (22). And there was also a study indicating that WSB2 expression was related to the resistance to prednisone and vincristine in myeloma (23). Besides, a study found that WSB2 was associated with poor clinical outcome of malignant fibrous histiocytoma (24). Moreover, a recent study suggested that WSB2 related to the overall survival time period of estrogen receptor (ER)-positive breast cancer patients with high risk scores (25). Additionally, knockdown of WSB2 also could suppress growth and metastasis through regulating p-Rb, CDK4 and cyclin D3 in melanoma cells (13). Consistent with existing reports, our results revealed that WSB2 was frequently elevated in lung cancer, especially in Xuanwei lung cancer tissues and cell lines. Knockdown of WSB2 notably reduced cell viability, cell invasion, EMT process, while induced apoptotic cell death and cell cycle arrest of Xuanwei lung cancer cells. Besides, we also found that knockdown of WSB2 delayed tumor growth in vivo. Our research supports that WSB2 acts as a crucial prognostic bio-marker of lung cancer, notably lung cancer in Xuanwei.
Accumulating evidence has reported the regulatory roles of Wnt/β-catenin signaling pathway in lung cancer. For instance, circular RNA-ITCH suppressed proliferation of lung cancer through inhibiting the Wnt/β-catenin pathway (26). Besides, there was a study that found that Wnt/β-catenin could regulate the stemness of lung cancer stem cell, such as, curcumin inhibited lung cancer stem cells through inactivation Wnt/β-catenin pathway (27). Wnt/β-catenin pathway was activated abnormally in lung cancer stem cells, and β-catenin knockdown effectively reduced lung cancer stem cells properties (28). Moreover, increasing evidence indicated that Wnt/β-catenin pathway was associated with the carcinogenesis of NSCLC, for example, a recent study found that FOXP3 facilitated the tumorigenesis and progression of NSCLC via modulating Wnt/β-catenin pathway (29). For example, SOX9 facilitated metastasis and EMT process through Wnt/β-catenin pathway (30). In view of the important role of Wnt/β-catenin pathway in carcinogenesis and progression (31). Accordingly, we also measured the levels of c-Myc and β-catenin in A549 and XWLC-05, and found that it was regulated by WSB2. Thus, based on all the findings, we demonstrated that WSB2 regulated Xuanwei lung cell growth and metastasis via regulating the Wnt/β-catenin signaling (Figure 5).
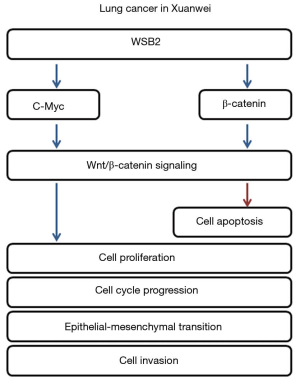
Conclusions
Taken together, current results suggest that WSB2 was augmented in lung cancer, particularly in Xuanwei lung cancer tissues and cells. Besides, overexpression of WSB2 also led to the activation of Wnt/β-catenin signaling. Furthermore, inhibition of Wnt/β-catenin signaling relieved the promotion of lung cancer caused by overexpression of WSB2. Therefore, our study found that WSB2 promoted the progression of lung cancer in Xuanwei by activation of Wnt/β-catenin signaling pathway, which might act as a therapeutic target for lung cancer, notably lung cancer in Xuanwei.
Acknowledgments
We thank all the authors who contributed to this study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2450
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-2450
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2450). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Yunnan Cancer Hospital (NO.: KY2019.57) and informed consent was taken from all the patients. The procedures for care and use of animals were approved by the Ethics Committee of the Third Affiliated Hospital of Kunming Medical University and all applicable institutional regulations and concerning the ethical use of animals were followed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li J, Guo W, Ran J, et al. Five-year lung cancer mortality risk analysis and topography in Xuan Wei: a spatiotemporal correlation analysis. BMC Public Health 2019;19:173. [Crossref] [PubMed]
- Liu XY, Liu LQ, Zou XN, et al. Epidemiological Features of Lung Cancer Mortality between 1990 and 2016 in Xuanwei City,Yunnan Province. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2019;41:338-43. [PubMed]
- Wang J, Duan Y, Meng QH, et al. Integrated analysis of DNA methylation profiling and gene expression profiling identifies novel markers in lung cancer in Xuanwei, China. PLoS One 2018;13:e0203155. [Crossref] [PubMed]
- Li R, Liu Y, Wang T, et al. The characteristics of lung cancer in Xuanwei County: A review of differentially expressed genes and noncoding RNAs on cell proliferation and migration. Biomed Pharmacother 2019;119:109312. [Crossref] [PubMed]
- Hilton DJ, Richardson R, Alexander W, et al. Twenty Proteins Containing a C-terminal SOCS Box Form Five Structural Classes. Proc Natl Acad Sci U S A 1998;95:114-9. [Crossref] [PubMed]
- Cao J, Wang Y, Dong R, et al. Hypoxia-Induced WSB1 Promotes the Metastatic Potential of Osteosarcoma Cells. Cancer Res 2015;75:4839-51. [Crossref] [PubMed]
- Shichrur K, Feinberg-Gorenshtein G, Luria D, et al. Potential role of WSB1 isoforms in growth and survival of neuroblastoma cells. Pediatr Res 2014;75:482-6. [Crossref] [PubMed]
- Sarraj MA, McClive J, Szczepny A, et al. Expression of Wsb2 in the developing and adult mouse testis. Reproduction 2007;133:753-61. [Crossref] [PubMed]
- Ram Makena M, Himavanth G, Dattesh V, et al. Wnt/β-Catenin Signaling: The Culprit in Pancreatic Carcinogenesis and Therapeutic Resistance. Int J Mol Sci 2019;20:4242. [Crossref] [PubMed]
- Li X, Lu Q, Xie W, et al. Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/β-Catenin signaling. Biochem Biophys Res Commun 2018;496:443-9. [Crossref] [PubMed]
- Rima M, Daghsni M, Lopez A, et al. Down-regulation of the Wnt/β-catenin signaling pathway by Cacnb4. Mol Biol Cell 2017;28:3699-708. [Crossref] [PubMed]
- Qu T, Zhao Y, Chen Y, et al. Down-regulated MAC30 expression inhibits breast cancer cell invasion and EMT by suppressing Wnt/β-catenin and PI3K/Akt signaling pathways. Int J Clin Exp Pathol 2019;12:1888-96. [PubMed]
- Zhang Y, Li Z, Zhao W, et al. WD repeat and SOCS box containing protein 2 in the proliferation, cycle progression, and migration of melanoma cells. Biomed Pharmacother 2019;116:108974. [Crossref] [PubMed]
- Zheng R, Zeng H, Zuo T, et al. Lung cancer incidence and mortality in China, 2011. Thorac Cancer 2016;7:94-9. [Crossref] [PubMed]
- Ren H, Cao W, Chen G, et al. Lung Cancer Mortality and Topography: A Xuanwei Case Study. Int J Environ Res Public Health 2016;13:473. [Crossref] [PubMed]
- Liu L, Liu X, Ma X, et al. Analysis of the associations of indoor air pollution and tobacco use with morbidity of lung cancer in Xuanwei, China. Sci Total Environ 2020;717:135232. [Crossref] [PubMed]
- Kim C, Chapman RS, Hu W, et al. Smoky coal, tobacco smoking, and lung cancer risk in Xuanwei, China. Lung Cancer 2014;84:31-5. [Crossref] [PubMed]
- Kang M, Shi J, Li B, et al. LncRNA DGCR5 regulates the non-small cell lung cancer cell growth, migration, and invasion through regulating miR-211-5p/EPHB6 axis. Biofactors 2019;45:788-94. [Crossref] [PubMed]
- Haque M, Kendal JK, MacIsaac RM, et al. WSB1: from homeostasis to hypoxia. J Biomed Sci 2016;23:61. [Crossref] [PubMed]
- Poujade FA, Mannion A, Brittain N, et al. WSB-1 regulates the metastatic potential of hormone receptor negative breast cancer. Br J Cancer 2018;118:1229-37. [Crossref] [PubMed]
- Jia YY, Zhao JY, Li BL, et al. miR-592/WSB1/HIF-1α axis inhibits glycolytic metabolism to decrease hepatocellular carcinoma growth. Oncotarget 2016;7:35257-69. [Crossref] [PubMed]
- Kim JJ, Lee SB, Yi SY, et al. WSB1 overcomes oncogene-induced senescence by targeting ATM for degradation. Cell Res 2017;27:274-93. [Crossref] [PubMed]
- Erkeland SJ, Aarts LH, Irandoust M, et al. Novel role of WD40 and SOCS box protein-2 in steady-state distribution of granulocyte colony-stimulating factor receptor and G-CSF-controlled proliferation and differentiation signaling. Oncogene 2007;26:1985-94. [Crossref] [PubMed]
- Daigeler A, Klein-Hitpass L, Stricker I, et al. Malignant fibrous histiocytoma--pleomorphic sarcoma, NOS gene expression, histology, and clinical course. A pilot study. Langenbecks Arch Surg 2010;395:261-75. Erratum in: Langenbecks Arch Surg 2009 May;394(3):589. Adrien, Daigeler [corrected to Daigeler, Adrien]; Ludger, Klein-Hitpass [corrected to Klein-Hitpass, Ludger];Ingo, Stricker [corrected to Stricker, Ingo]; Oliver, Müller [corrected to Müller, Oliver]; Cornelius, Kuhnen [corrected to Kuhnen, Cornelius]; M. [Crossref] [PubMed]
- Zhou H, Lv Q, Guo Z. Transcriptomic signature predicts the distant relapse in patients with ER+ breast cancer treated with tamoxifen for five years. Mol Med Rep 2018;17:3152-7. [PubMed]
- Wan L, Zhang L, Fan K, et al. Circular RNA-ITCH Suppresses Lung Cancer Proliferation via Inhibiting the Wnt/β-Catenin Pathway. Biomed Res Int 2016;2016:1579490. [Crossref] [PubMed]
- Zhu JY, Yang X, Chen Y, et al. Curcumin Suppresses Lung Cancer Stem Cells via Inhibiting Wnt/β-catenin and Sonic Hedgehog Pathways. Phytother Res 2017;31:680-8. [Crossref] [PubMed]
- Zhu J, Jiang Y, Yang X, et al. Wnt/β-catenin pathway mediates (-)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem Biophys Res Commun 2017;482:15-21. [Crossref] [PubMed]
- Yang S, Liu Y, Li MY, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer 2017;16:124. [Crossref] [PubMed]
- Huang JQ, Wei FK, Xu XL, et al. SOX9 drives the epithelial-mesenchymal transition in non-small-cell lung cancer through the Wnt/β-catenin pathway. J Transl Med 2019;17:143. [Crossref] [PubMed]
- Shi L, Wu YX, Yu JH, et al. Research of the relationship between β-catenin and c-myc-mediated Wnt pathway and laterally spreading tumors occurrence. Eur Rev Med Pharmacol Sci 2017;21:252-7. [PubMed]



