
Co-expression of VEGF-C and survivin predicts poor prognosis in esophageal squamous cell carcinoma
Introduction
Esophageal cancer (EC) is the eighth most common malignant tumor and the sixth most leading cause of cancer death worldwide (1,2). China has the highest morbidity and mortality from EC in the world, particularly from esophageal squamous cell carcinoma (ESCC) (1,3). The average number of new EC cases is about 223,000 per year, with 150,000 deaths (1). There are radiotherapy, chemotherapy and surgery, the main treatment for EC (4). Even with surgery, the 5-year survival rate is low, mainly due to recurrence and metastasis (5). For the locally advanced ESCC, the clinical trial NEOCRTEC5010 demonstrated that neoadjuvant chemoradiotherapy improves survival over surgery alone (6). Growing evidence has indicated that prognostic biomarkers, including carbohydrate antigen 72-4 (CA72-4), cytokeratin 19 fragment antigen 21-1 (Cyfra21-1) and carbohydrate antigen 19-9 (CA19-9) were reported to be crucial in diagnosis and prognosis in ESCC (7). Lymphatic involvement is the main route of EC metastasis and one of the prognostic factors in ESCC (8). Identifying a factor that can be used for early detection of lymph node metastasis to predict the prognosis of ESCC patients is essential. Some studies have indicated that vascular endothelial growth factor-C (VEGF-C) and survivin, a member of the inhibitor of apoptosis protein (IAP) family, may be involved in lymphatic metastasis.
VEGF-C is a stimulator of lymphatic endothelial cells. Multiple studies have demonstrated a relationship between VEGF-C, its receptors [vascular endothelial growth factor receptor 3 (VEGFR-3)], and micro-lymphatic vessel density (MLVD) (8), suggesting that VEGF-C and VEGFR-3 are important factors in inducing lymphangiogenesis (9,10) and promoting cancer metastasis (11,12). Survivin can promote cell proliferation and inhibit cell apoptosis (13). It is a key factor in tumor angiogenesis, metastasis (14,15), and progression (16). Studies in oral squamous cell carcinoma and breast, esophageal, and gastric cancer have demonstrated a significant relationship between survivin expression and lymphatic metastasis (17-19) and identified survivin as a prognostic factor. Furthermore, the expression of both VEGF-C and survivin has been positively correlated with lymph node invasion (20). Zhang et al. (21) demonstrated that HIF-1α, survivin and VEGF were positive correlation in EC, suggesting that they may play a synergistic role in the occurrence and development of EC. However, studies on the relationship between VEGF-C and survivin and their combined role in determining the prognosis and survival of ESCC patients are scarce. The current study aimed to analyze the potential link between the co-expression of VEGF-C and survivin and clinicopathological features, particularly the influence of VEGF-C and survivin co-expression on the prognosis of ESCC patients. This study also investigated whether the analysis VEGF-C and survivin co-expression could be used as a feasible and effective marker to predict the prognosis and survival of ESCC patients. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2498).
Methods
Patients
A total of 97 patients at the Shantou University Medical College Cancer Hospital were enrolled in this study from May 2014 to April 2016. Inclusion criteria was defined as: patients with ESCC without surgical treatment and distant metastasis. Exclusion criteria was those who did not meet the above inclusion criteria. There were 78 males and 19 females, ranging from 43 to 75 years of age, with a mean age of 59.28 years and a median age of 60 years. According to the infiltration depth, eight patients had pT1 tumors, nine had pT2 tumors, 79 had pT3 tumors, and one patient had a pT4 tumor. Tumor metastasis to the lymph nodes was detected in 56 cases (57.7%). Among the 97 cases, 38 were stage N1, 14 were stage N2, four were stage N3, and the remaining 41 cases were stage N0. In total, there were 33 cases with high differentiation, 54 with medium differentiation, and ten with low differentiation. The median follow-up time was 37.6 (range, 6.9 to 60) months. The time from the date of definitive surgery to the date of death due to any cause or the date of the last follow-up was defined as overall survival (OS).
Experimental reagents
The VEGF-C and VEGFR-3 polyclonal antibodies were purchased from Beijing Zhongshang Biotechnology Co., Ltd. (Beijing, China). The survivin and Ki-67 polyclonal antibodies, ready-to-use immunohistochemistry (IHC) MaxVisionTM secondary antibody, and 3,3'-diaminobenzidine (DAB) kit were purchased from Fuzhou Maixin Biotechnology Development Co., Ltd. (Fuzhou, China).
IHC
All samples were fixed in 10% neutral buffered formalin for 8 to 48 h, followed by dehydration with alcohol and xylene. The dehydrated samples were embedded in paraffin. Immunohistochemical staining was performed using the MaxVision two-step method. Briefly, tissues were cut into 4-µm sections. The sections were baked at 65 °C for 1 h, deparaffinized with xylene, and rehydrated with an ethanol gradient. The sections were incubated with 0.3% H2O2 for 10 min to block endogenous peroxidases, followed by antigen retrieval (VEGF-C, survivin, and Ki-67 with pH 6.0, 0.01 M citrate buffer, high-pressure repair; VEGFR-3 with pH 9.0 EDTA, high-temperature repair). After allowing the samples to cool at room temperature, they were washed three times with phosphate-buffered saline (PBS) for 3 min. The sections were incubated with primary antibody for 1 h at 37 °C and secondary antibody at 37 °C for 15 min. Finally, the sections were stained for 5 min with DAB and counterstained with hematoxylin for 1 min. The slides were sealed with neutral gum. Negative controls were incubated with PBS buffer instead of the primary antibodies.
IHC analysis
Stained slides were analyzed by two pathologists in a blinded fashion. Positive cellular staining was brownish-yellow. Negative staining was defined as no significant difference in color intensity from that of the background. Staining was visualized by light microscopy. For analysis, the strongest stained area was selected under low power (50×), and then ten visual fields were observed under high power (400×). The staining for 100 cells was scored for each patient sample.
VEGF-C, VEGFR-3, and survivin staining were scored using a semi-quantitative scoring system, as previously described (22-24). Briefly, scoring was performed according to the intensity of specific staining: 0, no staining; 1, weak staining; 2, intermediate staining; 3, strong staining. In addition, the percentage of positive cells within the total cells counted was semi-quantitatively scored as follows: 0, negative; 1, 1% to 10% positive; 2, 11% to 50% positive; 3, >50%. Finally, the intensity and percentage scores were multiplied to yield the immunohistochemical score. An immunohistochemical score of ≥3 was considered positive. Specifically, the final immunohistochemical scores were defined as follows: 3 to 4, weakly positive (+); 5 to 7, moderately positive (++); 8 to 9, strongly positive (+++). Ki-67 staining was analyzed as the percentage of Ki-67-positive cells in the total tumor cells.
Statistical analysis
All data were analyzed using SPSS 19.0 statistical software. The measurement data are presented as the mean ± standard deviation (). The counting data are expressed as the rate using Pearson’s χ2 test. Spearman used for correlation analysis. Survival curves were assessed using the Kaplan-Meier method, and the data were compared using the log-rank test. Univariate and multivariate analyses used to determine the impact of the variables on patient survival. Two-sided P values <0.05 were considered statistically significant. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by medical ethics committee of the Cancer Hospital of Shantou University Medical College (No. 2019039). The informed consent is not required for this study.
Results
Correlation between the expression of VEGF-C, VEGFR-3 and survivin and clinicopathological features of ESCC
To investigate whether VEGF-C, VEGFR-3, survivin, and Ki-67 were expressed in ESCC, we performed IHC on tumors from patients with ESCC. We observed that VEGF-C, VEGFR-3, and survivin were mainly located in the cytoplasm of cancer cells and interstitial cells that were surrounding the cancer nest. The staining was brownish-yellow, granular, and focal or diffusely distributed in the marginal part of the cancer nest. Survivin was occasionally present in the nucleus, whereas Ki-67 was specifically localized in the nucleus. Representative IHC images of VEGF-C, VEGFR-3, survivin were presented in Figure 1. The positive rates of VEGF-C, VEGFR-3, and survivin expression were 64.9% (63/97), 51.5% (50/97), and 67.0% (65/97), respectively. As shown in Table 1, positive VEGF-C expression was associated with advanced T stage (70.0% vs. 41.2%, P=0.024), more advanced N stage (100.0% vs. 85.7% vs. 68.4% vs. 48.8%, P=0.038), and lymph node metastasis (75.0% vs. 51.2%, P=0.015). However, there were no statistical differences between male and female groups, <60- and ≥60-year-old age groups, perineural invasion negative and positive groups, or well-differentiation and poor differentiation groups (P>0.05).
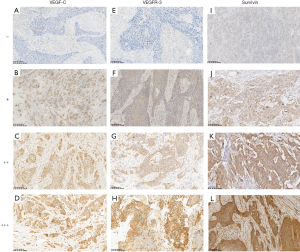
Table 1
| Features | N | VEGF-C positive | VEGFR-3 positive | Survivin positive | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | % | χ2 | P | Case | % | χ2 | P | Case | % | χ2 | P | ||||
| Gender | |||||||||||||||
| Male | 78 | 51 | 65.4 | 0.033 | 0.855 | 40 | 51.3 | 0.011 | 0.916 | 54 | 69.2 | 0.888 | 0.346 | ||
| Female | 19 | 12 | 63.2 | 10 | 52.6 | 11 | 57.9 | ||||||||
| Age (y) | |||||||||||||||
| <60 | 47 | 30 | 63.8 | 0.500 | 0.823 | 26 | 55.3 | 0.520 | 0.471 | 29 | 61.7 | 1.162 | 0.281 | ||
| ≥60 | 50 | 33 | 66.0 | 24 | 48.0 | 36 | 72.0 | ||||||||
| T stage | |||||||||||||||
| ≤T2 | 17 | 7 | 41.2 | 5.117 | 0.024 | 7 | 41.2 | 0.887 | 0.346 | 7 | 41.2 | 6.223 | 0.013 | ||
| >T2 | 80 | 56 | 70.0 | 43 | 53.8 | 58 | 72.5 | ||||||||
| N stage | |||||||||||||||
| N0 | 41 | 20 | 48.8 | 8.406 | 0.038 | 14 | 34.1 | 10.558 | 0.014 | 21 | 51.2 | 10.289 | 0.016 | ||
| N1 | 38 | 26 | 68.4 | 22 | 57.9 | 31 | 81.6 | ||||||||
| N2 | 14 | 12 | 85.7 | 11 | 78.6 | 9 | 64.3 | ||||||||
| N3 | 4 | 4 | 100.0 | 3 | 75.0 | 4 | 100.0 | ||||||||
| Lymph node metastasis | |||||||||||||||
| Negative | 41 | 21 | 51.2 | 5.880 | 0.015 | 14 | 34.1 | 8.609 | 0.003 | 21 | 51.2 | 8.010 | 0.005 | ||
| Positive | 56 | 42 | 75.0 | 36 | 64.3 | 44 | 78.6 | ||||||||
| Perineural invasion | |||||||||||||||
| Negative | 87 | 57 | 65.5 | 0.120 | 0.729 | 45 | 51.7 | 0.011 | 0.918 | 56 | 64.4 | 2.666 | 0.103 | ||
| Positive | 10 | 6 | 60.0 | 5 | 50.0 | 9 | 90.0 | ||||||||
| Differentiation | |||||||||||||||
| Low | 10 | 8 | 80.0 | 1.114 | 0.573 | 6 | 60.0 | 0.412 | 0.814 | 10 | 100.0 | 6.225 | 0.044 | ||
| Medium | 54 | 34 | 63.0 | 28 | 51.9 | 36 | 66.7 | ||||||||
| High | 33 | 21 | 63.6 | 16 | 48.5 | 19 | 57.6 | ||||||||
Statistically significant (P<0.05) values are in bold. VEGF-C, vascular endothelial growth factor-C; VEGFR-3, vascular endothelial growth factor receptor 3; ESCC, esophageal squamous cell carcinoma.
The correlations between survivin and clinicopathological characteristics shown in Table 1. Positive survivin expression was associated with advanced T stage (72.5% vs. 41.2%, P=0.013), more advanced node stage (100.0% vs. 64.3% vs. 81.6% vs. 51.2%, P=0.016), lymph node metastasis (78.6% vs. 51.2%, P=0.005), and worse differentiation (100.0% vs. 66.7% vs. 57.6%, P=0.044). There were no differences between the male and female groups, <60- and ≥60-year-old age groups, or perineural invasion-negative and -positive groups (P>0.05). Positive VEGFR-3 expression was associated with more advanced lymph node status (75.0% vs. 78.6% vs. 57.9% vs. 34.1%, P=0.014) and lymph node metastasis (64.3% vs. 34.1%, P=0.003). However, there were no statistical differences between the male and female groups, <60- and ≥60-year-old age groups, P ≤ T2 and P > T2 groups, perineural invasion-negative and -positive groups, or well differentiation and poor differentiation groups (P>0.05).
Association of co-expression of VEGF-C and survivin with pathological characteristics of ESCC
As shown in Table 2, survivin expression was positively correlated with VEGF-C (rs=0.280, P=0.006) and VEGFR-3 (rs=0.286, P=0.005) in the 97 ESCC tissues. Based on the expression patterns of VEGF-C and survivin, the enrolled patients were classified into four groups: VEGF-C (–)/survivin (–), (V–S–); VEGF-C (+)/survivin (–), (V+S–); VEGF-C (–)/survivin (+), (V–S+); VEGF-C (+)/survivin (+), (V+S+). We found that the frequency of V+S+ was higher in advanced T stage (P<0.001). As shown in Table 3, there was a significant association of the V+S+ group with lymph node involvement. Patients with N3 lymph node metastasis had a significant higher proportion of V+S+ (100%) than N0–2 patients (29.3%/52.6%/57.1%) (rs=0.336, P=0.001). Co-expression of VEGF-C and survivin was associated with a lower level of differentiation (rs=–0.204, P=0.0045). However, there were no statistical differences between the V+S+ and other groups in terms of gender, age, or perineural invasion (P>0.05). As shown in Table 4, the V+S+ group had significantly higher Ki-67 levels (proliferation index) compared to the other groups (V–S–/V+S–/V–S+) (rs=0.230, P=0.024).
Table 2
| Biomarkers | N | Survivin | rs | P | |||
|---|---|---|---|---|---|---|---|
| – | + | ++ | +++ | ||||
| VEGF-C | |||||||
| – | 34 | 13 | 20 | 1 | 0 | 0.280 | 0.006 |
| + | 39 | 13 | 18 | 7 | 1 | ||
| ++ | 23 | 6 | 7 | 9 | 1 | ||
| +++ | 1 | 0 | 0 | 1 | 0 | ||
| VEGFR-3 | |||||||
| – | 47 | 20 | 21 | 5 | 1 | 0.286 | 0.005 |
| + | 40 | 12 | 19 | 9 | 0 | ||
| ++ | 9 | 0 | 4 | 4 | 1 | ||
| +++ | 1 | 0 | 1 | 0 | 0 | ||
Statistically significant (P<0.05) values are in bold. VEGF-C, vascular endothelial growth factor-C; VEGFR-3, vascular endothelial growth factor receptor 3; ESCC, esophageal squamous cell carcinoma.
Table 3
| Features | V–S– (n=13) | V+S– (n=19) | V–S+ (n=21) | V+S+ (n=44) | χ2 | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | % | Case | % | Case | % | Case | % | ||||||
| Gender | |||||||||||||
| Male | 10 | 12.8 | 15 | 19.2 | 17 | 21.8 | 37 | 47.4 | 0.775 | 0.855 | |||
| Female | 3 | 15.8 | 5 | 26.3 | 4 | 21.1 | 7 | 36.8 | |||||
| Age (y) | |||||||||||||
| <60 | 7 | 14.9 | 11 | 23.4 | 10 | 21.3 | 19 | 40.4 | 0.325 | 0.723 | |||
| ≥60 | 6 | 12.0 | 8 | 16.0 | 11 | 22.0 | 25 | 50.0 | |||||
| T stage | |||||||||||||
| ≤T2 | 8 | 47.1 | 2 | 11.8 | 2 | 11.8 | 5 | 29.4 | 20.152 | <0.001 | |||
| >T2 | 5 | 6.3 | 17 | 21.3 | 19 | 23.8 | 39 | 48.8 | |||||
| N stage | |||||||||||||
| N0 | 11 | 26.8 | 9 | 22.0 | 9 | 22.0 | 12 | 29.3 | 20.515 | 0.015 | |||
| N1 | 1 | 2.6 | 6 | 15.8 | 11 | 28.9 | 20 | 52.6 | 0.336* | 0.001# | |||
| N2 | 1 | 7.1 | 4 | 28.6 | 1 | 7.1 | 8 | 57.1 | |||||
| N3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 100.0 | |||||
| Lymph node metastasis | |||||||||||||
| Negative | 11 | 26.8 | 9 | 22.0 | 9 | 22.0 | 12 | 29.3 | 13.814 | 0.003 | |||
| Positive | 2 | 3.6 | 10 | 17.9 | 12 | 21.4 | 32 | 57.1 | |||||
| Perineural invasion | |||||||||||||
| Negative | 13 | 14.9 | 18 | 20.7 | 17 | 19.5 | 39 | 44.8 | 3.805 | 0.283 | |||
| Positive | 0 | 0.0 | 1 | 10.0 | 4 | 40.0 | 5 | 45.4 | |||||
| Differentiation | |||||||||||||
| Low | 0 | 0.0 | 0 | 0.0 | 2 | 20.0 | 8 | 80.0 | 7.589 | 0.270 | |||
| Medium | 7 | 13.0 | 11 | 20.4 | 13 | 24.1 | 23 | 42.6 | –0.204* | 0.045# | |||
| High | 6 | 18.2 | 8 | 24.2 | 6 | 18.2 | 13 | 39.4 | |||||
Statistically significant (P<0.05) values are in bold. *, using spearman test, rs value; #, using spearman test, P value. VEGF-C, vascular endothelial growth factor-C; ESCC, esophageal squamous cell carcinoma; V–S–, VEGF-C (–)/survivin (–); V+S–, VEGF-C (+)/survivin (–); V–S+, VEGF-C (–)/survivin (+); V+S+, VEGF-C (+)/survivin (+).
Table 4
| Factors | N | Ki-67 (%), mean ± SD | rs | P |
|---|---|---|---|---|
| VEGF-C | ||||
| Negative | 34 | 53.67±23.68 | 0.162 | 0.112 |
| Positive | 63 | 61.90±18.54 | ||
| Survivin | ||||
| Negative | 32 | 54.53±19.19 | 0.187 | 0.066 |
| Positive | 65 | 61.23±21.26 | ||
| Case | ||||
| V–S– | 13 | 50.00±17.91 | 0.230 | 0.024 |
| V+S– | 19 | 57.63±19.89 | ||
| V–S+ | 21 | 55.95±26.82 | ||
| V+S+ | 44 | 63.75±17.85 |
Statistically significant (P<0.05) values are in bold. VEGF-C, vascular endothelial growth factor-C; ESCC, esophageal squamous cell carcinoma; SD, standard deviation; V–S–, VEGF-C (–)/survivin (–); V+S–, VEGF-C (+)/survivin (–); V–S+, VEGF-C (–)/survivin (+); V+S+, VEGF-C (+)/survivin (+).
Co-expression of VEGF-C and survivin predicts worse prognosis in ESCC
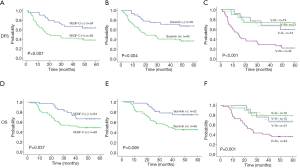
The follow-up period for all patients of the study ranged from 6.9 to 60 months, with a median duration of 37.6 months. Of the 97 ESCC patients, 42 patients died during the follow-up period. The remaining 55 patients were followed until July 2020. Factors, including patient gender, age, N stage, T stage, lymph node metastasis, perineural invasion, differentiation, VEGF-C, VEGFR-3 and survivin expression, and VEGF-C and survivin co-expression groups (V–S–/V+S–/V–S+/V+S+), were subjected to univariate and multivariate analyses for disease-free survival (DFS) and OS. The survival analysis is presented in Tables 5,6 and Figure 2. Univariate analysis showed that six variables (greater N stage, lymph node metastasis-positive, VEGF-C-positive, VEGFR-3-positive, survivin-positive, and the V+S+ group) had a worse prognosis. Gender, age, T stage, perineural invasion, and differentiation were not correlated with DFS or OS. DFS was significantly worse in the VEGF-C-positive patients compared to VEGF-C-negative patients (P=0.007, Figure 2A). Similarly, survival was significantly worse for the survivin-positive patients compared to the survivin-negative patients (P=0.004, Figure 2B). The V+S+ group had a worse prognosis than the other groups (V–S–/V+S–/V–S+) (P<0.001, Figure 2C). As for the OS, OS was significantly worse in the VEGF-C-positive patients compared to VEGF-C-negative patients (P=0.037, Figure 2D). Similarly, survival was significantly worse for the survivin-positive patients compared to the survivin-negative patients (P=0.009, Figure 2E). The V+S+ group had a worse prognosis than the other groups (V–S–/V+S–/V–S+) (P=0.001, Figure 2F). Most importantly, the multivariate DFS and OS analysis demonstrated that N stage, co-expression of VEGF-C and survivin were independent prognostic factors for ESCC (DFS: 95% CI: 1.254 to 2.324, P=0.001; 95% CI: 1.147 to 2.220, P=0.006; OS: 95% CI: 1.142 to 2.205, P=0.006; 95% CI: 1.080 to 2.193, P=0.017) (Tables 5,6).
Table 5
| Prognostic factors | Univariate analyses | Multivariate analyses | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Gender | |||||||
| Male/female | 0.960 | 0.480–1.921 | 0.908 | ||||
| Age (y) | |||||||
| <60/≥60 | 0.897 | 0.515–1.562 | 0.701 | ||||
| T stage | |||||||
| ≤T2/>T2 | 1.791 | 0.763–4.204 | 0.181 | ||||
| N stage | |||||||
| N0/N1/N2/N3 | 1.989 | 1.478–2.678 | <0.001 | 1.707 | 1.254–2.324 | 0.001 | |
| Lymph node metastasis | |||||||
| Negative/positive | 2.566 | 1.382–4.764 | 0.003 | ||||
| Perineural invasion | |||||||
| Negative/positive | 1.683 | 0.755–3.750 | 0.203 | ||||
| Differentiation | |||||||
| Low/medium/high | 0.794 | 0.516–1.222 | 0.294 | ||||
| VEGF-C | |||||||
| Negative/positive | 2.382 | 1.243–4.567 | 0.009 | ||||
| VEGFR-3 | |||||||
| Negative/positive | 1.778 | 1.009–3.134 | 0.047 | ||||
| Survivin | |||||||
| Negative/positive | 2.675 | 1.336–5.356 | 0.005 | ||||
| Case | |||||||
| V+S+/V+S–/V–S+/V–S– | 1.822 | 1.323–2.509 | <0.001 | 1.596 | 1.147–2.220 | 0.006 | |
Statistically significant (P<0.05) values are in bold. DFS, disease-free survival; ESCC, esophageal squamous cell carcinoma; VEGF-C, vascular endothelial growth factor-C; VEGFR-3, vascular endothelial growth factor receptor 3; V–S–, VEGF-C (–)/survivin (–); V+S–, VEGF-C (+)/survivin (–); V–S+, VEGF-C (–)/survivin (+); V+S+, VEGF-C (+)/survivin (+).
Table 6
| Prognostic factors | Univariate analyses | Multivariate analyses | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Gender | |||||||
| Male/female | 0.850 | 0.394–1.833 | 0.172 | ||||
| Age (y) | |||||||
| <60/≥60 | 0.915 | 0.503–1.665 | 0.772 | ||||
| T stage | |||||||
| ≤T2/>T2 | 1.788 | 0.704–4.544 | 0.222 | ||||
| N stage | |||||||
| N0/N1/N2/N3 | 1.836 | 1.341–2.513 | <0.001 | 1.587 | 1.142–2.205 | 0.006 | |
| Lymph node metastasis | |||||||
| Negative/positive | 2.261 | 1.161–4.406 | 0.016 | ||||
| Perineural invasion | |||||||
| Negative/positive | 1.421 | 0.599–3.373 | 0.426 | ||||
| Differentiation | |||||||
| Low/medium/high | 0.777 | 0.489–1.234 | 0.284 | ||||
| VEGF-C | |||||||
| Negative/positive | 2.046 | 1.030–4.063 | 0.041 | ||||
| VEGFR-3 | |||||||
| Negative/positive | 1.933 | 1.041–3.588 | 0.037 | ||||
| Survivin | |||||||
| Negative/positive | 2.721 | 1.261–5.870 | 0.011 | ||||
| Case | |||||||
| V+S+/V+S–/V–S+/V–S– | 1.746 | 1.240–2.459 | 0.001 | 1.539 | 1.080–2.193 | 0.017 | |
Statistically significant (P<0.05) values are in bold. OS, overall survival; ESCC, esophageal squamous cell carcinoma; VEGF-C, vascular endothelial growth factor-C; VEGFR-3, vascular endothelial growth factor receptor 3; V–S–, VEGF-C (–)/survivin (–); V+S–, VEGF-C (+)/survivin (–); V–S+, VEGF-C (–)/survivin (+); V+S+, VEGF-C (+)/survivin (+).
Discussion
Tumor spread and metastasis are the leading causes of treatment failure. Lymphatic metastasis is one of the primary means of metastasis and a major factor in the prognosis of patients with ESCC. VEGF-C is a member of the VEGF family and well known for its involvement in the selective proliferation of lymphatic vessels during oncogenesis and progression (25,26). VEGF-C enhances cell proliferation, growth, and invasion, and promotes angiogenesis, tumor metastasis, and progression by activating the tyrosine kinase receptor VEGFR-3 (27). The VEGF-C/VEGFR3 signaling pathway promotes cell migration and invasion, thereby inducing tumor metastasis. High VEGF-C expression levels are associated with shorter survival time in breast (28) and lung (29,30) cancer, and ESCC (31). Melanoma patients with lymph node involvement have high VEGF-C expression (32). In addition, VEGFR-3 serum levels are significantly higher in ESCC patients than in healthy donors, suggesting that VEGFR-3 may be a valuable diagnostic marker for ESCC (33). In the current study, we evaluated the VEGF-C and VEGFR-3 expression levels in 97 ESCC patient samples by IHC and found that 64.9% of the samples were VEGF-C-positive, and 51.5% were VEGFR-3-positive. These results were consistent with previous studies (34). We found that VEGF-C was associated with T and N stage but not gender, age, perineural invasion, or differentiation. In addition, our results showed that the positive rate of VEGF-C expression in the negative lymph node metastasis group was significantly lower than that in the positive group.
Survivin is a member of the IAP family that is a significant regulator of cell proliferation and apoptosis (35,36). Survivin inhibits caspase-3 and caspase-7 activity and the processing of caspase-9, thereby preventing cell apoptosis (37). In this study, we demonstrated that 67.0% of the ESCC tumors were survivin-positive, which was similar to previously reported data (38). Survivin expression was associated with T and N stage, lymph node metastasis, and differentiation but not gender, age, or perineural invasion.
High VEGF-C or survivin expression levels are related to lymph node involvement and positively correlated with each other in papillary thyroid carcinoma (39). In breast cancer, VEGF-C and survivin are highly expressed and also positively correlated (40). The co-expression of VEGF-C and survivin is positively correlated with positive lymph node, while downregulation of survivin can decrease VEGF-C expression and reduce lymphatic metastasis and invasion and is associated with a reduction in breast cancer mortality (20). In gastric cancer cells, expression of survivin and VEGF-C are significantly associated with lymph node metastasis (41). Survivin is considered to be a regulator of VEGF-C in gastric cells, and gastric cancer patients with co-expression of survivin and VEGF-C usually have a poor prognosis. In ESCC, the exact mechanism of co-expression of VEGF-C and survivin has not yet been delineated. In our study, VEGF-C positively correlated with survivin. Furthermore, we found that co-expression of VEGF-C and survivin was associated with advanced T stage, lymph node involvement, more advanced node status, and worse differentiation. It was not statistically related to gender, age, or perineural invasion. More importantly, patients with N3 lymph node metastasis had a significant higher proportion of V+S+ (100%) than N0–2 patients (P=0.001).
Nuclear proliferating antigen (Ki-67), is expressed in all phases of the cell cycle, except G0. It is one of the most reliable indicators of tumor cell proliferation and used to predict tumor invasion and prognosis (42). Our results showed that although VEGF-C and survivin expression levels were not correlated with Ki-67, V+S+ patients (i.e., expressing both VEGF-C and survivin) had a higher positive rate for Ki-67 compared to the other expression groups (V–S–/V+S–/V–S+). Thus, ESCC occurrence may be related to an interaction between VEGF-C and survivin.
In this study, we found that patients with positive VEGF-C or survivin expression had significantly worse DFS and OS than those with negative expression. These results are consistent with previous studies that showed that overexpression of VEGF-C, VEGFR-3, or survivin was associated with poor prognosis (8,43-45). In this study, we firstly demonstrated that ESCC patients with overexpression of both VEGF-C and survivin (V+S+ group) had a worse prognosis than those in the other co-expression groups (V–S–/V+S–/V–S+). Furthermore, co-expression of VEGF-C and survivin, but not the expression of the individual genes, was an independent prognostic factor in ESCC patients.
In summary, this study found that VEGF-C and survivin overexpressed in ESCC tissues with lymph node involvement. Overexpression of VEGF-C or survivin may predict poor prognosis. Co-expression of both these factors predicts a worse prognosis in ESCC. In particular, our results identified the co-expression of VEGF-C and survivin as a potential prognostic marker for ESCC. More in-depth studies on the molecular mechanisms underlying the relationship between these two biomarkers and their roles in angiogenesis and metastasis in ESCC are required to fully understand their importance in this disease.
Acknowledgments
Thanks to BioMed Proofreading LLC (USA) for Language Editing.
Funding: This work partly supported by the funds from
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2498
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-2498
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2498). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by medical ethics committee of the Cancer Hospital of Shantou University Medical College (No. 2019039). The informed consent is not required for this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stewart BW, Wild CP. World cancer report 2014. Geneva: International Agency for Research on Cancer, 2014.
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [Crossref] [PubMed]
- Luo ML, Shen XM, Zhang Y, et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res 2006;66:11690-9. [Crossref] [PubMed]
- Batra R, Malhotra GK, Singh S, et al. Managing squamous cell esophageal cancer. Surg Clin North Am 2019;99:529-41. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Yang Y, Huang X, Zhou L, et al. Clinical use of tumor biomarkers in prediction for prognosis and chemotherapeutic effect in esophageal squamous cell carcinoma. BMC Cancer 2019;19:526. [Crossref] [PubMed]
- Omoto I, Matsumoto M, Okumura H, et al. Expression of vascular endothelial growth factor-C and vascular endothelial growth factor receptor-3 in esophageal squamous cell carcinoma. Oncol Lett 2014;7:1027-32. [Crossref] [PubMed]
- Albrecht I, Christofori G. Molecular mechanisms of lymphangiogenesis in development and cancer. Int J Dev Biol 2011;55:483-94. [Crossref] [PubMed]
- Tao S, Yu J, Xu Y, et al. PC4 induces lymphangiogenesis dependent VEGF-C/VEGF-D/VEGFR-3 axis activation in lung adenocarcinoma. Am J Cancer Res 2015;5:1878-89. [PubMed]
- He M, Cheng Y, Li W, et al. Vascular endothelial growth factor C promotes cervical cancer metastasis via up-regulation and activation of RhoA/ROCK-2/moesin cascade. BMC Cancer 2010;10:170. [Crossref] [PubMed]
- Alessi C, Scapulatempo Neto C, Viana CR, et al. PD-1/PD-L1 and VEGF-A/VEGF-C expression in lymph node microenvironment and association with melanoma metastasis and survival. Melanoma Res 2017;27:565-72. [Crossref] [PubMed]
- Ryan BM, O'Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev 2009;35:553-62. [Crossref] [PubMed]
- Su C. Survivin in survival of hepatocellular carcinoma. Cancer Lett 2016;379:184-90. [Crossref] [PubMed]
- Zhao G, Wang Q, Wu Z, et al. Ovarian primary and metastatic tumors suppressed by survivin knockout or a novel survivin inhibitor. Mol Cancer Ther 2019;18:2233-45. [Crossref] [PubMed]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 2008;8:61-70. [Crossref] [PubMed]
- Al-Joudi FS, Iskandar ZA, Hasnan J, et al. Expression of survivin and its clinicopathological correlations in invasive ductal carcinoma of the breast. Singapore Med J 2007;48:607-14. [PubMed]
- Kim YH, Kim SM, Kim YK, et al. Evaluation of survivin as a prognostic marker in oral squamous cell carcinoma. J Oral Pathol Med 2010;39:368-75. [PubMed]
- Da CL, Xin Y, Zhao J, et al. Significance and relationship between Yes-associated protein and survivin expression in gastric carcinoma and precancerous lesions. World J Gastroenterol 2009;15:4055-61. [Crossref] [PubMed]
- Cai X, Ma S, Gu M, et al. Survivin regulates the expression of VEGF-C in lymphatic metastasis of breast cancer. Diagn Pathol 2012;7:52. [Crossref] [PubMed]
- Zhang HZ, Zhang J, Xu N, et al. Expression and clinical significance of hypoxia inducible factor-1alpha, survivin and vascular endothelial growth factor in esophageal squamous cell carcinoma. Zhonghua Bing Li Xue Za Zhi 2007;36:689-90. [PubMed]
- Yang Z, Wang YG, Su K. VEGF-C and VEGF-D expression and its correlation with lymph node metastasis in esophageal squamous cell cancer tissue. Asian Pac J Cancer Prev 2015;16:271-4. [Crossref] [PubMed]
- Shawber CJ, Funahashi Y, Francisco E, et al. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J Clin Invest 2007;117:3369-82. [Crossref] [PubMed]
- Perrouin Verbe MA, Bruyere F, Rozet F, et al. Expression of store-operated channel components in prostate cancer: the prognostic paradox. Hum Pathol 2016;49:77-82. [Crossref] [PubMed]
- Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997;276:1423-5. [Crossref] [PubMed]
- Yeh YW, Cheng CC, Yang ST, et al. Targeting the VEGF-C/VEGFR3 axis suppresses Slug-mediated cancer metastasis and stemness via inhibition of KRAS/YAP1 signaling. Oncotarget 2017;8:5603-18. [Crossref] [PubMed]
- Sun P, Gao J, Liu YL, et al. RNA interference (RNAi)-mediated vascular endothelial growth factor-C (VEGF-C) reduction interferes with lymphangiogenesis and enhances epirubicin sensitivity of breast cancer cells. Mol Cell Biochem 2008;308:161-8. [Crossref] [PubMed]
- Bando H, Weich HA, Horiguchi S, et al. The association between vascular endothelial growth factor-C, its corresponding receptor, VEGFR-3, and prognosis in primary breast cancer: a study with 193 cases. Oncol Rep 2006;15:653-9. [Crossref] [PubMed]
- Kalitin NN, Karamysheva AF. RARalpha mediates all-trans-retinoic acid-induced VEGF-C, VEGF-D, and VEGFR3 expression in lung cancer cells. Cell Biol Int 2016;40:456-64. [Crossref] [PubMed]
- Bi MM, Shang B, Wang Z, et al. Expression of CXCR4 and VEGF-C is correlated with lymph node metastasis in non-small cell lung cancer. Thorac Cancer 2017;8:634-41. [Crossref] [PubMed]
- Su CM, Su YH, Chiu CF, et al. Vascular endothelial growth factor-C upregulates cortactin and promotes metastasis of esophageal squamous cell carcinoma. Ann Surg Oncol 2014;21:S767-75. [Crossref] [PubMed]
- Lund AW, Duraes FV, Hirosue S, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep 2012;1:191-9. [Crossref] [PubMed]
- Su Mer A, Demir A, Kemik AS, et al. Serum vascular endothelial growth factor receptor-3 levels in patients with esophageal squamous cell cancer. Ann Ital Chir 2017;88:20-25. [PubMed]
- Kumagai Y, Tachikawa T, Higashi M, et al. Vascular endothelial growth factors C and D and lymphangiogenesis at the early stage of esophageal squamous cell carcinoma progression. Dis Esophagus 2018; [Crossref] [PubMed]
- Ahmed MB, Shehata HH, Moussa M, et al. Prognostic significance of survivin and tumor necrosis factor-alpha in adult acute lymphoblastic leukemia. Clin Biochem 2012;45:112-6. [Crossref] [PubMed]
- McKenzie JA, Grossman D. Role of the apoptotic and mitotic regulator survivin in melanoma. Anticancer Res 2012;32:397-404. [PubMed]
- Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 1998;58:5315-20. [PubMed]
- Kato J, Kuwabara Y, Mitani M, et al. Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer 2001;95:92-5. [Crossref] [PubMed]
- Selemetjev S, Savin S, Paunovic I, et al. Concomitant high expression of survivin and vascular endothelial growth factor-C is strongly associated with metastatic status of lymph nodes in papillary thyroid carcinoma. J Cancer Res Ther 2018;14:S114-9. [Crossref] [PubMed]
- Li X, Dang X, Sun X. Expression of survivin and VEGF-C in breast cancer tissue and its relation to lymphatic metastasis. Eur J Gynaecol Oncol 2012;33:178-82. [PubMed]
- Zhang J, Zhu Z, Sun Z, et al. Survivin gene expression increases gastric cancer cell lymphatic metastasis by upregulating vascular endothelial growth factor-C expression levels. Mol Med Rep 2014;9:600-6. [Crossref] [PubMed]
- Ahmed A, Al-Tamimi DM. Incorporation of p-53 mutation status and Ki-67 proliferating index in classifying Her2-neu positive gastric adenocarcinoma. Libyan J Med 2018;13:1466573. [Crossref] [PubMed]
- Li C, Zhu M, Lou X, et al. Transcriptional factor OCT4 promotes esophageal cancer metastasis by inducing epithelial-mesenchymal transition through VEGF-C/VEGFR-3 signaling pathway. Oncotarget 2017;8:71933-45. [Crossref] [PubMed]
- Xia H, Chen S, Huang H, et al. Survivin over-expression is correlated with a poor prognosis in esophageal cancer patients. Clin Chim Acta 2015;446:82-5. [Crossref] [PubMed]
- Xia H, Shen J, Chen S, et al. Overexpression of VEGF-C correlates with a poor prognosis in esophageal cancer patients. Cancer Biomark 2016;17:165-70. [Crossref] [PubMed]


