
CXCR3 expression as a prognostic factor in gastric cancer: a meta-analysis
Introduction
Gastric cancer is the fourth most prevalent cancer, and the second most common cause of cancer-related mortality worldwide (1,2). The prognosis of gastric cancer is rather unfavorable, with a five-year survival rate below 30% (2). Genetically and biologically, gastric cancer is a heterogeneous disorder, and several prognostic factors have been suggested, but their use is limited (3).
CXC chemokine receptor 3 (CXCR3) is a G-protein-coupled receptor shown to influence immune responses, generation of the vascular system, and the repair of wounds (4). Recently, CXCR3 has been shown to play a crucial role in tumorigenesis, and the expression of CXCR3 is associated with the prognosis of many cancers, such as colorectal, breast and ovarian cancer, renal cell carcinoma, and glioblastoma (5-11).
Although CXCR3 expression as a prognostic factor has been studied in gastric cancer, it has been demonstrated in smaller samples and has not been systematically assessed (12-16). Therefore, we performed a meta-analysis to establish the prognostic significance of CXCR3 expression in gastric cancer.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2862).
Methods
Literature search
We searched the literature using the following keywords: (CXCR3) and (cancer or tumor or carcinoma) and (prognostic or prognosis or survival). The search was conducted on June 1, 2020 using PubMed, Embase, and the Cochrane library, and the manual search was performed at the same time.
Selection criteria
It was included in the analysis only if the following criteria were met in the literature: (I) CXCR3 expression was identified using immunohistochemistry in the human cancer tissue of the stomach, (II) the hazard ratio (HR) and 95% confidence interval (CI) were established between CXCR3 expression and patient survival. Excluded were duplicated literature, reviews, conference abstracts, case reports, and non-English articles.
Data collection and quality assessment
The entire literature contained in the analysis was reviewed to gather the primary data for each literature. The primary data collected included the following: first author, year of publication, country, sample size, sex of patients, study and follow-up period, and CXCR3 expression cut-off. Two authors compiled the data, and differences were revised through consensus.
Quality evaluation was conducted on the literature included in the analysis, and the Newcastle-Ottawa Scale was used for quality evaluation. Quality evaluation was also performed independently by two authors, and disagreement resolution was revised through consensus.
Statistical analysis
Mata-analysis was conducted using StataSE12 (Stata, College Station, TX, USA). Pooled HR with 95% CI: was calculated to evaluate the correlations between CXCR3 expression and patient survival, and the I2 value was used to determine the heterogeneity between the included literature. The funnel plot was plotted to verify publication bias visually. Egger’s test was performed to validate the funnel plot’s statistical significance. Sensitivity analysis was performed to determine the reliability of the pooled results. Pooled odds ratio (OR) with 95% CI: was calculated to assess the associations between CXCR3 expression and clinicopathological factors. Statistical significance was defined as a P<0.05.
Results
Search results and primary data of studies
Figure 1 illustrates the process of selecting the included literature. All studies were published in China, and the study period was from 2008 to 2013. For a total of 716, the number of samples varied from 96 to 192. The included studies’ quality evaluation score was between six and eight, indicating good quality (Table 1).
Table 1
| Study | Country | Sample size | Gender (male/female) | Study period | Median follow-up (months) | Clinical outcome | CXCR3 detection | Cut-off value of CXCR3 expression | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al. [2019] | China | 156 | 114/42 | 2008–2013 | 21.5 | OS | IHC | Staining scores with intensity and extent (≥2) | 8 |
| Chen et al. [2018] | China | 169 | 124/45 | 2008–2013 | 22 | OS | IHC | Moderate staining and more than 25% of cells staining positive (≥2) | 8 |
| Zhou et al. [2016] | China | 103 | 72/31 | 2006–2010 | NA | OS | IHC | Positive staining | 6 |
| Hu et al. [2015] | China | 96 | 69/27 | 2008–2013 | NA | OS | IHC | Moderate staining and more than 25% of cells staining positive (≥2) | 7 |
| Li et al. [2015] | China | 192 | 138/54 | 2008–2013 | NA | OS | IHC | Moderate staining and more than 25% of cells staining positive (≥2) | 7 |
CXCR3, CXC chemokine receptor 3; IHC, immunohistochemistry; NA, not available; NOS, Newcastle-Ottawa Scale; OS, overall survival.
Association between high expression of CXCR3 and overall survival (OS)
The included studies’ heterogeneity was so considerable that the pooled HR was calculated using a random-effects model (I2=62.7%, P=0.030). The results revealed that the elevated expression of CXCR3 was associated with a favorable OS in gastric cancer (HR 0.46, 95% CI: 0.30–0.71, P<0.001) (Figure 2).

Association between high expression of CXCR3 and clinicopathological factors
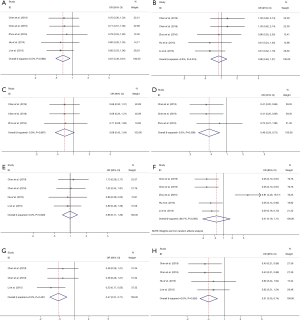
High CXCR3 expression was strongly associated with younger age (OR 0.67, 95% CI: 0.49–0.91, P=0.011), lower tumor grade (OR 0.46, 95% CI: 0.29–0.73, P=0.001), absence of lymph node metastasis (OR 0.47, 95% CI: 0.31–0.71, P<0.001), and lower Tumor-Node-Metastasis (TNM) stage (OR 0.51, 95% CI: 0.35–0.74, P<0.001). However, no substantial association was identified with sex, tumor size, Lauren classification, and tumor stage (Table 2) (Figure 3A,B,C,D,E,F,G,H).
Table 2
| Factor | Number of studies | Number of patients | Pooled OR (95% CI) |
P value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 (%) | P value | Model | |||||
| Age (old vs. young) | 5 | 716 | 0.67 (0.49–0.91) | 0.011 | 0.0 | 0.983 | Fixed |
| Gender (male vs. female) | 5 | 716 | 0.90 (0.63–1.27) | 0.541 | 0.0 | 0.418 | Fixed |
| Tumor size (≥5 vs. <5 cm) | 3 | 428 | 0.69 (0.45–1.04) | 0.077 | 0.0 | 0.997 | Fixed |
| Tumor grade (PD vs. WD MD) | 3 | 428 | 0.46 (0.29–0.73) | 0.001 | 0.0 | 0.599 | Fixed |
| Lauren classification (diffuse vs. intestinal) | 4 | 613 | 0.98 (0.71–1.36) | 0.914 | 0.0 | 0.949 | Fixed |
| Tumor stage (III IV vs. I II) | 5 | 716 | 0.57 (0.19–1.71) | 0.315 | 88.7 | <0.001 | Random |
| Lymph node metastasis (present vs. absent) | 3 | 517 | 0.47 (0.31–0.71) | <0.001 | 0.0 | 0.427 | Fixed |
| TNM stage (high vs. low) | 4 | 613 | 0.51 (0.35–0.74) | <0.001 | 0.0 | 0.826 | Fixed |
CI, confidence interval; CXCR3, CXC chemokine receptor 3; MD, moderately-differentiated; OR, odds ratio; PD, poorly-differentiated; TNM, tumor-node-metastasis; WD, well-differentiated.
Publication bias
According to the funnel plot, it appeared that there were small study effects, but Egger’s test did not prove it (P=0.232) (Figure 4A). The filled funnel plot also showed that the data did not change (HR 0.46, 95% CI: 0.30–0.71, P<0.001) (Figure 4B).

Sensitivity analysis
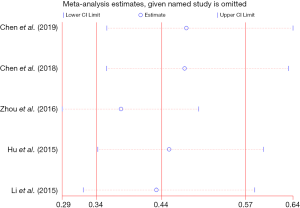
Sensitivity analysis indicated that the study published by Zhou et al. (15) affected the overall results (HR 0.38, 95% CI: 0.29–0.50) (Figure 5). However, the overall results were not substantially different (HR 0.44, 95% CI: 0.34–0.57), even after each study was excluded (Figure 5).
Discussion
This study showed a strong association between high expression of CXCR3 and a better prognosis than low expression in gastric cancer. We also demonstrated a correlation between CXCR3 expression and clinicopathological factors, which revealed a significant association between high CXCR3 expression and younger age, lower tumor grade, absent lymph node metastasis, and lower TNM stage.
CXCR3 is an interferon-inducible chemokine receptor expressed in various cells (17). CXCR3 comprises three isoforms, CXCR3-A, CXCR3-B, and CXCR3-Alt, in humans with distinct roles in cell biology and tumorigenesis (17). Of these, CXCR3-A and CXCR3-B are two well-studied isoforms (18). Cancer cells can control the expression of CXCR3 isoforms in a way that benefits overall and can decrease their proliferation and survival by overexpressing CXCR3-B and suppressing CXCR-A (18). Indeed, Hu et al. (16) demonstrated that CXCR3-B mRNA was substantially higher than CXCR3-A mRNA in gastric cancer tissue. CXCR3-B mRNA was significantly lower in patients with metastasis than in patients without metastasis. Therefore, our findings that high expression of CXCR3 indicates a favorable prognosis may have been derived from increased CXCR3-B in gastric cancer tissue. However, the mechanism underlying CXCR3 isoform expression remains to be elucidated, so further research is needed.
This analysis has some limitations. First, the number of studies included was small, making it challenging to analyze in various ways, including subgroup analysis. Second, all the included studies were published in China, so we need to consider whether this could apply to other regions.
However, in this study, we first presented the significance of CXCR3 expression as a prognostic factor in gastric cancer.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2862
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2862). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018;10:239-248. [Crossref] [PubMed]
- Verbeke H, Geboes K, Van Damme J, et al. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta 2012;1825:117-29. [PubMed]
- Durães C, Almeida GM, Seruca R, et al. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Archiv 2014;464:367-78. [Crossref] [PubMed]
- Ma B, Khazali A, Wells A. CXCR3 in carcinoma progression. Histol Histopathol 2015;30:781-92. [PubMed]
- Bai M, Chen X, Ba Y. CXCL10/CXCR3 overexpression as a biomarker of poor prognosis in patients with stage II colorectal cancer. Mol Clin Oncol 2016;4:23-30. [Crossref] [PubMed]
- Kawada K, Hosogi H, Sonoshita M, et al. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 2007;26:4679-88. [Crossref] [PubMed]
- Wu Z, Han X, Yan J, et al. The prognostic significance of chemokine receptor CXCR3 expression in colorectal carcinoma. Biomed Pharmacother 2012;66:373-7. [Crossref] [PubMed]
- Mulligan AM, Raitman I, Feeley L, et al. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res 2013;19:336-46. [Crossref] [PubMed]
- Windmüller C, Zech D, Avril S, et al. CXCR3 mediates ascites-directed tumor cell migration and predicts poor outcome in ovarian cancer patients. Oncogenesis 2017;6:e331. [Crossref] [PubMed]
- Rezakhaniha B, Dormanesh B, Pirasteh H, et al. Immunohistochemical distinction of metastases of renal cell carcinoma with molecular analysis of overexpression of the chemokines CXCR2 and CXCR3 as independent positive prognostic factors for the tumorigenesis. IUBMB Life 2016;68:629-33. [Crossref] [PubMed]
- Pu Y, Li S, Zhang C, et al. High expression of CXCR3 is an independent prognostic factor in glioblastoma patients that promotes an invasive phenotype. J Neurooncol 2015;122:43-51. [Crossref] [PubMed]
- Chen F, Yin S, Niu L, et al. Expression of the chemokine receptor CXCR3 correlates with dendritic cell recruitment and prognosis in gastric cancer. Genet Test Mol Biomarkers 2018;22:35-42. [Crossref] [PubMed]
- Chen F, Yuan J, Yan H, et al. Chemokine Receptor CXCR3 Correlates with Decreased M2 Macrophage Infiltration and Favorable Prognosis in Gastric Cancer. Biomed Res Int 2019;2019:6832867. [Crossref] [PubMed]
- Li K, Zhu Z, Luo J, et al. Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol 2015;8:14725-32. [PubMed]
- Zhou H, Wu J, Wang T, et al. CXCL10/CXCR3 axis promotes the invasion of gastric cancer via PI3K/AKT pathway-dependent MMPs production. Biomed Pharmacother 2016;82:479-88. [Crossref] [PubMed]
- Hu M, Li K, Maskey N, et al. Overexpression of the chemokine receptor CXCR3 and its correlation with favorable prognosis in gastric cancer. Hum Pathol 2015;46:1872-80. [Crossref] [PubMed]
- Kuo PT, Zeng Z, Salim N, et al. The role of CXCR3 and its chemokine ligands in skin disease and cancer. Front Med (Lausanne) 2018;5:271. [Crossref] [PubMed]
- Reynders N, Abboud D, Baragli A, et al. The distinct roles of CXCR3 variants and their ligands in the tumor microenvironment. Cells 2019;8:613. [Crossref] [PubMed]



