
“Double hit” B-lymphoblastic lymphoma with concurrent IGH/BCL2 and 8q24/MYC translocations: a case report
Introduction
The term “double-hit” lymphoma (DHL) was introduced to describe a small group of aggressive lymphomas with co-occurrence of two oncogenic translocations, such as MYC, BCL2, and/or BCL6 (1). The most common DHLs that harbor a MYC rearrangement at chromosome 8q24 and a rearrangement in chromosome 18q21 account for an estimated 2% of all B-cell malignancies and have a heterogeneous histology with a dismal outcome (2). Morphologically, these cases typically either resemble “B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL)” (most frequently), DLBCL, or less commonly bear a blastoid appearance (3). Among them, B-lymphoblastic lymphomas (B-LBLs) with a documented “double-hit” (DH) (IGH/BCL2 and 8q24/MYC rearrangement) are rare and their clinical, cytogenetic and immunophenotypic features have not been well elucidated. Here we report such a case of DH B-LBL. We present the following article in accordance with the CARE reporting checklist (available at: http://dx.doi.org/10.21037/tcr-20-2748).
Case presentation
A 39-year-old man complained of abdominal distention at admission in our hospital. Nothing special was found in the physical exam, and the patient denied of any past medical history or family history. A full-body CT scan revealed multiple lesions of retroperitoneal soft tissue density wrapping surrounding vessels and ureter. Dilation of left renal pelvis and upper ureter, hydronephrosis and hydroureter indicated malignancy. Pelvic effusion was notable with multiple adenopathy in enterocoelia (max size: 3.3 cm × 1.2 cm) and thickened peritoneum, which suggested the possibility of metastasis. PET/CT revealed mildly increased glucose uptake in most lymph node territories (SUV max =3.5), suggesting multiple involvement by lymphoma. Lactate dehydrogenase (LDH) was modestly elevated (300 U/L, normal range: 109–245 U/L). Blood routine did not display any anomaly with hemoglobin 141 g/L, platelets 295×109/L, and WBC 6.3×109/L.A core needle biopsy of the retroperitoneal soft tissue was then performed. Histologic examination yielded a diffuse infiltration of lymphoblasts varying from small-size to intermediate-size with round nuclei, which focally formed single files surrounded by fibrotic septa. These cells have high nuclear to cytoplasmic ratio, fine chromatin, and no inconspicuous nucleoli. Necrosis and apoptotic bodies were common. Mitotic figures were easily found. Prominent starry-sky appearance was not observed (Figure 1A).
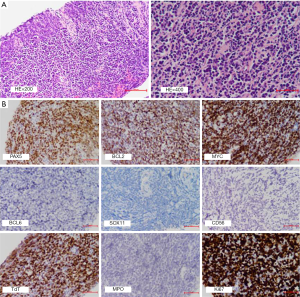
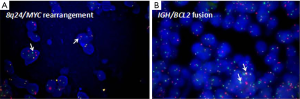
Immunophenotypic data regarding the cells of interest were derived from IHC with CD20–, PAX5+, CD79a+, CD10+, BCL2+ (70%), MYC+ (70%), BCL6–, MUM1–, CD30–, CD21–, Cyclin-D1–, SOX11–, CD56–, CD123–, TdT+, CD99+, MPO–, CD34–, CD117–, CD68 sparsely+, CD5 (±), CD3 (±); Ki-67 stained about 80% of the cells (Figure 1B). In situ hybridization for Epstein–Barr virus encoded RNA (EBER ISH) was negative. Subsequent bone marrow examination showed normal proliferation of hematopoietic cell without lymphoma involvement. Fluorescence in situ hybridization (FISH) performed on formalin-fixed paraffin-embedded (FFPE) mass tissues identified breakpoints near the distal 5’ end of MYC (Figure 2A), and breakpoints within the IGH locus and proximal to BCL2 gene (Figure 2B). The percentage of nuclei which scored positive for the MYC break was about 10% on the whole slice, and 80% for IGH/BCL2 fusion, respectively. The patient bore normal chromosome karyotype. Immunoglobulin gene arrangement analysis was not available due to the limited amount of tissue. Accordingly, a diagnosis of DH B-LBL was established based on the morphology, immunophenotype, and FISH analysis. After receiving six cycles of Hyper-CVAD, the patient’s symptoms alleviated and stayed alive at the last follow-up (2020.4.10, six months after the diagnosis). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion and conclusion
Lymphomas harboring concurrent IGH-BCL2/t (14;18)(q32;q21) and MYC/8q24 translocations, which are referred to as ‘‘double-hit’’ or ‘‘dual-hit’’ lymphomas (DHL), are a group of B-cell malignancies with a heterogeneous cytologic and histologic features. Rearrangements in MYC and BCL2 are extremely rare in B-LBL (4,5). Such DH B-LBL cases may emerge de novo or through transformation of follicular lymphomas, both of which have been described to have an aggressive clinical course and poor prognosis (2,6).
In Subramaniyam et al.’s study, the patients with de novo DH B-LBL were adults with a median age of 59 years. They had complex chromosomal abnormalities, in addition to the BCL2 and MYC translocations. The reported median overall survival was 1.5 months (range, <1–11 months) (4). Kelemen et al. also found the prognosis of B-LBL with a combined IGH/BCL2 and MYC translocation was poor, with a median survival of five months after the initial diagnosis (range, 3–27 months) (7). The present case was alive at the last follow-up (2020.4.10, six months after the diagnosis).
As to those TdT-positive neoplasms derived from a minority of follicular lymphomas (FL) and characterized by blastoid morphology, the current recommendation in the WHO-2016 scheme is to classify them as B-LBL transformed from follicular lymphoma, which is exceedingly rare as well, with fewer than 10 reported cases. Interestingly, most of these cases were featured with MYC rearrangement in addition to IGH/BCL2 fusion (2,6,8-13). Lymphoblastic transformation of FL is thus proposed to have occurred due to a gain of MYC rearrangement at an early stage of FL lymphomagenesis [immature B-precursor carrying t (14;18)(q32;q21) in the bone marrow], and then the expression of TdT has been interpreted as either a “dedifferentiation” to a lymphoblastic (pre-B-cell) stage of B-cell development or as a re-expression of TdT in B cells due to accumulated somatic hypermutations, a hallmark of germinal center B cells (1,8-10,12,14,15).
Although TdT is critical in discriminating between TdT-positive LBL and TdT-negative aggressive B cell lymphomas, exceptional cases of either double-hit or triple-hit high grade B cell lymphoma with an aberrant expression of TdT have been reported, which could be quite a diagnostic pitfall. Morphology, immunophenotype and key genetic profiles may facilitate the differential diagnoses (8,16,17).
DH B-LBL should also be distinguished from entities like blastic plasmacytoid dendritic cell neoplasm (BPDCN), blastoid variant of mantle cell lymphoma (MCL), and myeloid neoplasm (3,18,19). Among them, the reported BPDCNs have occurred mainly in elderly with a slight male predominance. The disease tends to involve multiple sites, most commonly the skin, followed by bone marrow and peripheral blood and lymph node. BPDCN is characterized by a diffuse, monomorphous infiltrate of medium-sized blast cells reminiscent of lymphoblasts or myeloblasts. The tumor cells express CD4, CD56, CD43, CD123 and lack expression of TdT, which distinct from LBL (3). Specific chromosomal aberrations are lacking, but complex karyotypes are common. Moreover, gene expression analysis of BPDCN reveals a unique signature, distinguished from those of myeloid and lymphoid acute leukemia (20).
As illustrated in the present case, strong expression of CD99 and TdT with lack of expression of CD20 in neoplastic cells has been used as features to support immaturity. Being positive for PAX5 and CD79a demonstrated B-cell differentiation. The age of the patient, the location of the lesion, and tumor cells being negative for CD123 and CD56 were not supportive of BPDCNs. MCL-associated markers (CyclinD1, SOX11) were negative, and MPO was not detected, excluding acute myeloid leukemia or B/myeloid acute leukemia, and blastoid variant of mantle cell lymphoma, respectively. Finally, a diagnosis of DH B-LBL was established based on the morphology, immunophenotype, and FISH analysis.
Of note, components of FL might be missed due to the limitations of biopsy. Theoretically, we cannot definitely rule out the possibility of lymphoblastic transformation of FL in this case. However, considering relatively low FDG uptake values and the patient denying oncologic history, there is insufficient evidence (either histologically or clinically) to support the setting of FL. De novo DH B-LBL was thus preferred.
At present, few guidelines regarding DH B-LBL are available in the literature or in the WHO scheme. The optimal clinical management of such patients has not been fully established attributed to the small number of reported cases. Multicenter data is warranted to better make clinical strategies on patients with DH B-LBL.
Acknowledgments
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2748
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2748). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood 2011;117:2319-31. [Crossref] [PubMed]
- Li S, Lin P, Fayad LE, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t (14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol 2012;25:145-56. [Crossref] [PubMed]
- Swerdlow S, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition. Lyon: IARC, 2017.
- Subramaniyam S, Fraser CR, Rao PH, et al. De novo B lymphoblastic leukemia/lymphoma in an adult with t (14;18)(q32;q21) and c-MYC gene rearrangement involving 10p13. Leuk Lymphoma 2011;52:2195-9. [Crossref] [PubMed]
- Liu W, Hu S, Konopleva M, et al. De Novo MYC and BCL2 Double-hit B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL) in Pediatric and Young Adult Patients Associated With Poor Prognosis. Pediatr Hematol Oncol 2015;32:535-47. [Crossref] [PubMed]
- Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol 2010;34:327-40. [Crossref] [PubMed]
- Kelemen K, Holden J, Johnson LJ, et al. Immunophenotypic and cytogenetic findings of B-lymphoblastic leukemia/lymphoma associated with combined IGH/BCL2 and MYC rearrangement. Cytometry B Clin Cytom 2017;92:310-4. [Crossref] [PubMed]
- Geyer JT, Subramaniyam S, Jiang Y, et al. Lymphoblastic transformation of follicular lymphoma: a clinicopathologic and molecular analysis of 7 patients. Hum Pathol 2015;46:260-71. [Crossref] [PubMed]
- Ok CY, Medeiros LJ, Thakral B, et al. High-grade B-cell lymphomas with TdT expression: a diagnostic and classification dilemma. Mod Pathol 2019;32:48-58. [Crossref] [PubMed]
- De Jong D, Voetdijk BM, Beverstock GC, et al. Activation of the c-myc oncogene in a precursor-B-cell blast crisis of follicular lymphoma, presenting as composite lymphoma. N Engl J Med 1988;318:1373-8. [Crossref] [PubMed]
- Gauwerky CE, Haluska FG, Tsujimoto Y, et al. Evolution of B-cell malignancy: pre-B-cell leukemia resulting from MYC activation in a B-cell neoplasm with a rearranged BCL2 gene. Proc Natl Acad Sci U S A 1988;85:8548-52. [Crossref] [PubMed]
- Kroft SH, Domiati-Saad R, Finn WG, et al. Precursor B-lymphoblastic transformation of grade I follicle center lymphoma. Am J Clin Pathol 2000;113:411-8. [Crossref] [PubMed]
- Kaplan A, Samad A, Dolan MM, et al. Follicular lymphoma transformed to "double-hit" B lymphoblastic lymphoma presenting in the peritoneal fluid. Diagn Cytopathol 2013;41:986-90. [Crossref] [PubMed]
- Mamessier E, Broussais-Guillaumot F, Chetaille B, et al. Nature and importance of follicular lymphoma precursors. Haematologica 2014;99:802-10. [Crossref] [PubMed]
- Young KH, Xie Q, Zhou G, et al. Transformation of follicular lymphoma to precursor B-cell lymphoblastic lymphoma with c-myc gene rearrangement as a critical event. Am J Clin Pathol 2008;129:157-66. [Crossref] [PubMed]
- Moench L, Sachs Z, Aasen G, et al. Double- and triple-hit lymphomas can present with features suggestive of immaturity, including TdT expression, and create diagnostic challenges. Leuk Lymphoma 2016;57:2626-35. [Crossref] [PubMed]
- Slot LM, Hoogeboom R, Smit LA, et al. B-Lymphoblastic Lymphomas Evolving from Follicular Lymphomas Co-Express Surrogate Light Chains and Mutated Gamma Heavy Chains. Am J Pathol 2016;186:3273-84. [Crossref] [PubMed]
- Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood 2008;111:5433-9. [Crossref] [PubMed]
- Ratei R, Hummel M, Anagnostopoulos I, et al. Common clonal origin of an acute B-lymphoblastic leukemia and a Langerhans' cell sarcoma: evidence for hematopoietic plasticity. Haematologica 2010;95:1461-6. [Crossref] [PubMed]
- Sapienza MR, Fuligni F, Agostinelli C, et al. Molecular profiling of blasticplasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 2014;28:1606-16. [Crossref] [PubMed]


