
Immune checkpoint blockade for lung cancer: state of the art
Introduction
Molecularly-guided therapy has revolutionized lung cancer with dramatic responses in patients with aberrations in EGFR, ALK, and ROS1 (1-3). However, the majority of patients with lung cancer (~70%), lack these targetable driver mutations and have historically been treated with cytotoxic chemotherapy (4). Despite lacking a targetable driver mutation, the majority of patients with lung cancer have an overall higher mutational burden more akin to melanoma, which presents an opportunity to unleash the host immune system against tumor neoantigens (5,6). With an increasing appreciation of the role the adaptive immune system plays in lung cancer, the development of therapies to target maladapted immunological pathways such as CTLA-4 and programmed cell death protein 1 (PD-1; CD279)/programmed death-ligand 1 (PD-L1; CD274) have ushered in a new era for these patients (7-9).
The central role the immune system plays in cancer has been known for over a century. In the 1890s, Dr. William Coley described a post-operative patient with sarcoma with residual disease at resection, who achieved a complete remission following two severe bacterial skin infections (10). One of the presumed biologic factors in that patient’s response is interleukin-2 (IL-2), which has since become a therapy with the potential of durable remissions in a minority (6-10%) of patients with advanced melanoma and renal cell cancer (RCC) (11). Further refinements in immunomodulation would require the discovery of immune checkpoints and their role in facilitating tumor escape, leading to the clinical development of novel immunotherapeutics targeting the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, CD152) and PD-1/PD-L1 immune checkpoints (8,9,12,13). To the surprise of many in the oncology community, early phase trials of immune checkpoint blockade demonstrated efficacy not only in the cancer histologies traditionally thought to be “immunogenic” (melanoma and RCC), but in non-small cell lung cancer (NSCLC) as well (7-9). Reviewed here are recent clinical developments in the field of immunotherapy in NSCLC with a focus on the published clinical efficacy of immune checkpoint blockade, predictive biomarkers for efficacy, and potential future directions utilizing immunotherapeutic combinations.
Immunobiology in NSCLC
Immune checkpoints, such as CTLA-4 and PD-1, are present on activated T cells as a means of immune homeostasis and to minimize the risk of incidental autoimmune attack due to persistent activation of T cells (12). The analogy of T cell activation as akin to starting an automobile is often used. The cognate T cell-antigen presenting cell interaction represents a “key and ignition” paired interaction where only a specific key can activate a particular automobile (Signal 1). Activating (accelerator) and inhibitory (brake) receptors on T cells modulate the strength of the response (Signal 2) based on interactions with the immune microenvironment. Inhibitory checkpoints CTLA-4, PD-1, and PD-L1/PD-L2, among others (13). Overexpression of inhibitory checkpoint ligands by tumors and recruitment of immunosuppressive cells into the tumor microenvironment modifies the tumor microenvironment towards an immunosuppressive state that favors tumor growth, a process termed “immunoediting” (14). Immunoediting is a maladaptive interaction between the host immune system and tumor, which results in the host immune system selecting for a less immunogenic tumor over time, and conversely, a tumor selecting for a less immunologically adept host immune microenvironment.
CTLA-4 is a centrally-acting inhibitory T cell checkpoint which acts on T cells residing in lymphoid organs (15). In contrast, PD-1 is an inhibitory T cell immune checkpoint involved in the peripheral effector phase of T-cell activation, leading to immune tolerance of cells that express PD-L1 and PD-L2 (16). Accordingly, PD-1 knockout mice have a milder autoimmune phenotype relative to CTLA-4 knockout mice (15,17). This finding parallels the clinical severity of observed toxicities to immune checkpoint blockade, which are more pronounced with anti-CTLA-4 therapy relative to anti-PD-1 therapy (18).
PD-L1 is expressed on a variety of somatic cells as well as on B cells, T cells, dendritic cells, macrophages, and mast cells (19). T-cell mediated cytolysis via interferon-gamma release leads to adaptive up-regulation of PD-L1 whereby normal mucosal cells create an immunologic exclusion zone to protect against autoimmune attack in the setting of chronic inflammation. Tumor cells co-opt this immune homeostatic mechanism, design to protect normal mucosa, and express PD-L1 to avoid immunologic surveillance to facilitate cancer growth. While a myriad of PD-L1 immunohistochemistry (IHC) assays with a variety of cutoff thresholds complicate analyses, generally 40-60% of archival NSCLC tumor specimens will have PD-L1 expression (20). Correlative analysis of tumor specimens from multiple clinical trials, utilizing different anti-PD-L1 antibodies with different thresholds of positivity, has generally shown that anywhere from 25-50% of NSCLC specimens are considered PD-L1 positive. The attractive underlying immunobiology of NSCLC has shown merit in clinical practice, with durable responses in select patients with metastatic NSCLC (21-23).
Clinical efficacy
Ipilimumab
Anti-CTLA-4 blockade with ipilimumab in combination with chemotherapy in first-line treatment of metastatic NSCLC provided some of the earliest evidence of the efficacy of immune checkpoint blockade (7). Patients received six cycles of carboplatin/paclitaxel chemotherapy. Those patients who received phased ipilimumab (4 cycles of ipilimumab administered starting with cycle 3 of chemotherapy) had improved progression-free survival (PFS) (5.1 vs. 4.1 and 4.2 months, respectively) compared to those patients receiving chemotherapy alone or 4 cycles of ipilimumab starting with cycle 1 of chemotherapy. Immune-related grade 3-4 toxicities, predominantly related to colitis, were seen in 15-20% of patients treated with ipilimumab. Overall, given the modest survival improvement and toxicity with ipilimumab-based therapy in NSCLC, alternative therapeutic strategies would require exploration.
Nivolumab
A phase 1 trial of nivolumab (Bristol-Myers Squibb; BMS-936558, ONO-4538), an anti-PD-1 antibody, demonstrated an 18% response rate in 122 patients with NSCLC (9). Of note was the durability of response, in which the majority of responding patients had response duration greater than 6 months (8/14 responding patients), with some responses lasting longer than 1 year (5/14 patients). Additionally, durable stable disease lasting greater than 6 months was observed in 7% of patients on this study. Nivolumab was well-tolerated overall, with grade 3/4 adverse event (AE) rate in 6% of patients in this phase 1 trial.
Published concurrently, a phase 1 clinical trial of BMS-936559, an anti-PD-L1 antibody, resulted in a 10.2% response rate in 75 patients with NSCLC (8). All responding patients sustained their response to at least 6 months, with an additional 8% of patients achieving stabilization of disease lasting greater than 6 months. BMS-936559 was well tolerated with a grade 3/4 toxicity rate of 5%. Given the impressive tolerability and durability of response with monotherapy in select patients with refractory NSCLC, further investigation of nivolumab, in particular, was pursued.
Nivolumab in squamous NSCLC
A multinational, single-arm, phase 2 trial of nivolumab in 117 patients with refractory squamous cell lung cancer (CheckMate 063) demonstrated similarly impressive activity (23). In this study, 15% of patients had an objective response to nivolumab, with a median duration of response of at least 6 months. Time to response (TTR) was 3.3 months, consistent with delayed responses observed in earlier clinical trials. An additional 26% of patients had durable stable disease with a median duration of 6 months. Therapy was generally well tolerated, with 17% grade 3/4 toxicity. Of note, 3% of patients on this study developed immune-related pneumonitis, generally managed with corticosteroids with resolution in 3-4 weeks. However, 4 of 6 patients who developed pneumonitis discontinued therapy permanently, and one patient may have had immune-related pneumonitis as a contributor to death while on study. The presence of durable responses in patients with refractory squamous NSCLC in this study led to a randomized control trial of nivolumab vs. docetaxel in this setting (CheckMate 017) (24). In this study, 272 patients were randomized with a primary endpoint of overall survival (OS). Patients treated with nivolumab had a median OS of 9.2 vs. 6 months with docetaxel. In the nivolumab cohort, 42% of patients were alive at 1-year vs. 24% in the docetaxel arm. The response rate was 20% in patients treated with nivolumab vs. 9% with docetaxel (P=0.008). The time to initial response was 2.2 months, and the median duration of response was not reached for the nivolumab group, with 63% of responders with ongoing response. Nivolumab was well tolerated with a 7% grade 3/4 AE rate (no grade 3/4 pneumonitis) with no on-treatment deaths. CheckMate 063 and 017 were the basis for the FDA-approval of nivolumab on March 4, 2015 for refractory squamous NSCLC in patients who had progressed on platinum-based therapy.
Nivolumab in nonsquamous NSCLC
A phase 1/2 trial evaluated nivolumab in 129 patients with refractory NSCLC in both squamous and nonsquamous subtypes (22). The objective response rate (ORR) was similar across histologic subtypes: 17.1% for all NSCLC, 16.7% for squamous NSCLC, and 17.6% for nonsquamous NSCLC across all doses. The OS rate at 3 years in treated patients was an unprecedented 27% in this highly refractory population—54.3% of patients had received three or more prior therapies—with ongoing responses. Of note, patients with EGFR mutations (n=12) had similar benefit (ORR =16.7%) relative to the general study population (ORR =17.1%) and patients with KRAS mutations (n=21; ORR =14.3%). Nivolumab was generally well-tolerated with a grade 3/4 AE rate of 4.7%, which consisted predominantly of pneumonitis. Twelve patients had immune-related pneumonitis—grade 1/2 in eight patients, and grade 3/4 in three patients (2.3%)—and one patient had fatal pneumonitis, occurring outside the date of formal safety analysis. Overall, this trial demonstrated the impressive durability of response to anti-PD-1 therapy, even in heavily pretreated patients.
Pembrolizumab in NSCLC
Pembrolizumab (Merck, formerly lambrolizumab or MK-3475), an anti-PD-1 antibody, was studied in a large phase 1 trial (KEYNOTE-001) with 495 NSCLC patients (21). All patients received pembrolizumab at either 2 or 10 mg/kg every 3 weeks. The ORR was 19.4% with a median duration of response of 12.5 months. For patients with PD-L1 expression on tumor cells >50%, which represented 23.2% of the study population, the ORR was 45.2%. Responses were durable with 84.4% of patients with sustained response at time of analysis and a median duration of response of 12.5 months in all patients. Patients with PD-L1 >50% had a median PFS of 6.3 vs. 3.7 months for all patients. Therapy was generally well tolerated with grade 3-5 events reported in 9.5% of patients. Pneumonitis was observed in 1.8% of patients (n=9), with one death. There were no differences in efficacy or AEs between dose levels.
Atezolizumab in NSCLC
Atezolizumab (Roche/Genentech; MPDL3280A) is an anti-PD-L1 antibody that was studied in a phase 1 trial across multiple histologies (25). In this trial, 53 patients with NSCLC (n=41 non-squamous; n=11 squamous) were treated with atezolizumab with an ORR of 23% (21% non-squamous; 27% squamous). PFS at 6 weeks was 45% (44% non-squamous, 46% squamous). PD-L1 expression on tumor infiltrating-immune cells (IC) was associated with response (P=0.015) in NSCLC as well as across tumor types (P=0.007), and had improved performance as a predictive biomarker relative to tumor PD-L1 expression. PD-L1 staining intensity was associated with response in NSCLC, with 83% of patients with the highest PD-L1 immune cell IHC score of 3 (IC3) having a response to therapy. In contrast, patients with IC2 levels of PD-L1 expression had a lower ORR with 43% limited to disease stabilization. Similarly, PFS at 6 months was associated with level of PD-L1 expression on IC. While 83% of NSCLC patients with IC3 levels of PD-L1 expression achieved a 6-month PFS endpoint, only 14.3% of patients with IC2 and 25.6% patients with IC1 reached this endpoint. Of note, no cases of grade 3-5 pneumonitis were observed in this study across histologies.
Summary of clinical experience
Immune checkpoint blockade, particularly with anti-PD-1/anti-PD-L1 directed therapy, has demonstrated impressive activity in select patients with refractory NSCLC. While ORR may range broadly from 16% to 83% based on patient characteristics including PD-L1 expression, the additional presence of durable stable disease associated with impressive survival endpoints (for example, 27% 3-year OS in refractory NSCLC) has set a new standard for NSCLC therapy (Table 1) (22,24,25). Immune-related pneumonitis is of particular concern with anti-PD-1 therapy, appearing in approximately 2% of patients. Increased vigilance and early intervention with steroid therapy may improve the outcome of patients with pneumonitis, akin to improvements made in management of immune-related colitis from ipilimumab-based therapy in melanoma (18,26).
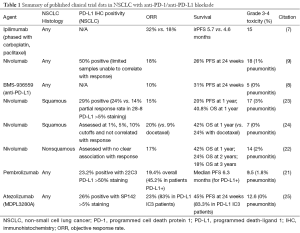
Full table
There are several unique clinical features of immune checkpoint blockade that are of note, particularly in juxtaposition to cytotoxic chemotherapy and targeted therapy. While responses are durable, radiographic responses can be delayed with TTR ranging from 2-6 months, depending on the study. Limited data suggest that patients with squamous histology (TTR 2-4 months) may achieve a response more rapidly than patients with nonsquamous (TTR 4-6 months) tumors, however further investigation into the molecular mechanisms behind this potential phenomenon (for example, neoantigen burden) will be required (22,24).
Furthermore, some patients may have unconventional immune-related responses (“pseudoprogression”) with initial radiographic progression followed by potential durable stable disease or response (27). However, pseudoprogression is generally rare in NSCLC patients treated with anti-PD-1 directed therapy (3-5% of patients), and patients with clear clinical progression (declining performance status, weight loss, and worsening clinical symptoms) should be switched to alternative therapy. This is unique from melanoma, and in particular with anti-CTLA-4 (ipilimumab, tremelimumab) therapy, which acts to recruit T cells into the tumor microenvironment which may radiographically appear as an enlarging lesion (28). Anti-PD-1/PD-L1 directed therapies predominantly act on immune cells already present within the tumor microenvironment. Moreover, NSCLC, a tumor that is overall less “immunogenic” than melanoma (potentially due to the quantity and quality of neoantigens produced by the tumor), likely results in decreased immune cell recruitment into the NSCLC microenvironment with a reduced rate of radiographic pseudoprogression (5). One common theme across histologies, aside from durability of response, is the ability for patients to recapture responses with retreatment, or to have continued responses off therapy (18,21,22,24). Overall, improved patient selection with the use of predictive biomarkers will maximize benefits, minimize risks, and help clarify treatment decision-making as anti-PD-1/anti-PD-L1 directed therapy use becomes more widespread.
Predictive biomarkers for response in NSCLC
Across histologic subtypes and trials in NSCLC, patients with tumors that are PD-L1 IHC positive seem to preferentially benefit from PD-1/PD-L1 directed therapy (20). While patients with PD-L1 IHC negative tumors may still derive benefit from therapy, patients with PD-L1 IHC positive tumors have a higher response rate and survival with anti-PD-1/PD-L1 directed therapy across studies (21,25,29). For example, pembrolizumab has been investigated in NSCLC, utilizing a 50% IHC cutoff for PD-L1 expression on tumor with the 22C3 assay. Based on this cutoff, 23% of tumors were positive for PD-L1 (>50% expression) and these patients had a 45.2% response rate, compared with an ORR of 19.5% in patients with PD-L1 expression 25-50%, an ORR of 12.9% in patients with PD-L1 expression of 1-24%, and an ORR of 6.1% in patients with PD-L1 expression <1% (21). Atezolizumab (MPDL3280A), an anti-PD-L1 antibody, has also been studied in NSCLC utilizing SP142 with 0-3+ grading (3+ for ≥10% cells, 2+ for ≥5 to <10% cells, 1+ for ≥1% to <5% cells; 0+ for <1%) and scoring of both tumor and immune cell PD-L1 expression (25). In this study, PD-L1 expression on IC was found to be more predictive than PD-L1 expression on tumor cells (TC). NSCLC patients with 3+ PD-L1 expression on immune cells (IC3) had an 83% response rate, compared with 14% with IC2 expression, 15% for IC1 expression, and 20% for IC0 expression. A similar trend in response rates associated with immune-cell PD-L1 expression was observed in other solid tumor types with this agent.
Data regarding PD-L1 IHC based on the 28-8 clone for nivolumab are mixed. In squamous NSCLC, PD-L1 IHC was not predictive of response with an ORR in the 15-21% range regardless of tumor PD-L1 expression (23,24). No relationship between PD-L1 IHC and response to nivolumab was observed in another NSCLC trial featuring both squamous and nonsquamous histologies (22). However, CheckMate 057, a phase 3 randomized control trial of nivolumab vs. docetaxel in advanced nonsquamous NSCLC showed a preferential benefit in patients with higher PD-L1 expression on tumor at the 1%, 5%, and 10% cutoffs (30). The reasons behind this disconnect are not clear, but may be related to technical issues related to sample collection and timing of biopsy, or biologic issues such as increased mutational burden in squamous NSCLC relative to nonsquamous tumors, which may overcome the predictive biomarker effect of PD-L1 IHC. Based on this limited data, it appears patients with NSCLC, and particularly patients with nonsquamous NSCLC, with higher levels of PD-L1 by IHC have superior responses to anti-PD-1/PD-L1 directed therapy. However, responses in PD-L1 IHC negative patients can be observed and may be related to biopsy site selection as well as timing of biopsy.
In addition to PD-L1 IHC, other biomarkers may determine which patients derive clinical benefit from anti-PD-1 directed therapy. Immune checkpoint blockade works through activation of existing antigen-specific T cells against the tumor. Thus, tumors with a high mutational burden are more likely to generate a neoantigen for which a cognate antigen-specific T cell exists. This T cell becomes activated in the setting of immune checkpoint blockade, resulting in the efficacy of the immune checkpoint inhibitor. Mechanistically, this has been shown to be the case in microsatellite unstable tumors with high mutational burden and an improved response to PD-1 blockade (6). Furthermore, identification of immunogenic neoantigens based on peptide prediction algorithms analyzing the tumor mutanome has been shown to generate individualized biomarkers for response to anti-CTLA-4 blockade in melanoma (31). A similar approach was taken with anti-PD-1 blockade in NSCLC (32). Of note, ORR and PFS were improved in patients with higher nonsynonymous mutational burden when treated with pembrolizumab. In particular, a molecular smoking signature based on genetic transversions showed that patients with transversion-high (TH) tumors had a higher ORR (56% vs. 17% in transversion-low tumors; P=0.03), durable clinical benefit rate (77% vs. 22%, P=0.004), and PFS. A molecular smoking signature more significantly correlated with response than clinical smoking history, and never smokers with mutations that resulted in higher mutational burden (e.g., POLD1, POLE, MSH2 mutations) had improved responses to pembrolizumab therapy, analogous to microsatellite-unstable gastrointestinal tumors (6).
Future directions
Immunotherapy, and in particular, immune checkpoint blockade, has revolutionized medical oncology and the care of patients with NSCLC. The promise of durable responses in select patients with NSCLC treated with immune checkpoint blockade, in particular anti-PD-1/PD-L1, has set a new bar for cancer therapy. Responses in 15-25% of NSCLC patients treated with anti-PD-1/anti-PD-L1 therapy are durable and can last years in select patients, even those with heavily pre-treated disease. Anti-PD-1/PD-L1 therapy is generally well-tolerated, though vigilance and early intervention on immune-related pneumonitis will be required as these therapies gain greater usage. More importantly, these therapies provide a solid base for combinatorial approaches utilizing targeted therapy, cellular therapy, as well as alternative modes of immunomodulation. Indeed, EGFR and ALK-aberrant NSCLC tumors can overexpress PD-L1, and combinatorial strategies combining EGFR and ALK inhibitors with anti-PD-1/anti-PD-L1 blockade are currently being tested in clinical trials (33,34). Concomitant inhibition of other immune checkpoints such as LAG3, TIM3, KIR, and BTLA may by synergistic with anti-PD-1 blockade and are under active investigation. Returning to the T cell as automobile analogy, this approach blocks multiple immunologic “brakes” leading to increased acceleration (activation). Additionally, immune costimulation via agonists of the tumor necrosis factor receptor superfamily (TNFRSF) such as OX40, 4-1BB, and GITR may represent an attractive combinatorial approach and are actively being studied (35). This approach is based on the premise that blocking an immunologic “brake” while pressing on an immunologic “accelerator,” may result in improved T cell responses against tumor.
Management of immune-related toxicity with combinatorial immunotherapeutics will be crucial, as has been demonstrated by combinations based on CTLA-4 blockade used in the treatment of melanoma (18). However, combinatorial toxicity may be driven by biology (e.g., CTLA-4 as a centrally acting checkpoint) and the development of predictive biomarkers may help guide therapeutic decision-making. For example, patients with metastatic melanoma who had PD-L1 negative tumors derived the greatest benefit from combinatorial ipilimumab plus nivolumab therapy, compared to nivolumab monotherapy (29). Similarly, NSCLC patients with tumors overexpressing PD-L1 tend to have superior responses to anti-PD-1/anti-PD-L1 therapy in most, but not all, clinical trials in NSCLC to date. Ultimately, improved predictive biomarkers will help determine which personalized immunotherapeutic combinations will maximize benefit, and minimize toxicity, for each individual cancer patient.
Lung cancer, and in particular NSCLC, has undergone a therapeutic revolution, with the adoption of molecular profiling and early intervention with targeted therapy for patients with driver mutations (36). The embrace of a precision medicine approach to NSCLC has led to the development of novel approaches such as cell-free DNA to assess for mechanisms of therapeutic resistance, and to the rational design of next-generation targeted therapies (37-39). The advent of immune-based therapies requires an expansion of this precision medicine approach to include not only molecular aberrations detected in tumor and in blood, but also to serial assessment of the tumor immune microenvironment. Assays such as PD-L1 IHC, mutational burden, neoantigen prediction, immune transcriptional signatures, T-cell receptor clonality, and others will require further investigation as they become increasingly integrated into clinical testing for therapeutic decision-making (20). Treatment of NSCLC is truly at a crossroads, with multiple potential paths for any particular patient, and the development and utilization of novel biomarkers will be needed in order to best guide patients through increasingly complex treatment decisions. With the advent of immunotherapy in NSCLC, and in particular, immune checkpoint blockade, another promising path has been discovered—and one that has just begun to be explored.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyudmila Bazhenova and Ajay Pal Singh Sandhu) for the series “Recent advances in radiotherapy and targeted therapies for lung cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.08.04). The series “Recent advances in radiotherapy and targeted therapies for lung cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [PubMed]
- Johnson BE, Kris MG, Berry LD, et al. A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas: The Lung Cancer Mutation Consortium (LCMC). J Clin Oncol 2013;31:abstr 8019.
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Parish CR. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol 2003;81:106-13. [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [PubMed]
- Patel SP, Osada T, Osada K, et al. Modulation of Immune System Inhibitory Checkpoints in Colorectal Cancer. Curr Colorectal Cancer Rep 2013;9:391-7.
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [PubMed]
- Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001;19:565-94. [PubMed]
- Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005;54:307-14. [PubMed]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219-42. [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [PubMed]
- Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007;8:239-45. [PubMed]
- Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 2015;14:847-56. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- Gettinger SN, Shepherd FA, Antonia SJ, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol 2014;32:abstr 8024.
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013;19:3936-43. [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [PubMed]
- Paz-Ares L, Horn L, Borghaei H, et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2015;33:15 suppl LBA109.
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [PubMed]
- Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A 2008;105:20852-7. [PubMed]
- Sundar R, Soong R, Cho BC, et al. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 2014;85:101-9. [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [PubMed]
- Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700-9. [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [PubMed]


