
Pulmonary tumor thrombotic microangiopathy of patient with both hepatocellular carcinoma and gastric signet-ring cell carcinoma: a case report and review of literature
Introduction
Pulmonary tumor thrombotic microangiopathy (PTTM), defined by von Harvey et al. (1) in 1990, is a rare pulmonary manifestation of malignancy. Tumor embolism, multiple microthrombi, and intimal myofibroblast proliferation in the pulmonary arteries and arterioles are among its histopathological characteristics (1,2). It is most commonly associated with gastric adenocarcinoma, but it has also been described in other carcinomas (1). The pathogenesis begins with the formation of microscopic tumor cell emboli that induce local activation of coagulation and fibrocellular intimal proliferation. Eventually, stenosis and/or occlusion occur along with an increase in pulmonary vascular resistance that results in PH, hemolytic anemia, and disseminated intravascular coagulation (DIC). It is associated with the development of clinical signs of pulmonary hypertension (PH) that result in acute or subacute cor pulmonale and subacute respiratory failure (1). Computed tomography (CT)-guided lung puncture biopsy or bronchoscopy lung biopsy is the gold standard for the diagnosis of PTTM. Elevated concentrations of D-dimer or fibrin degradation products, PH measured by cardiac ultrasound or right heart catheterization of the pulmonary artery, and distal perfusion abnormalities of pulmonary perfusion imaging may help clinicians establish an early diagnosis of PTTM (3). Further, no arterial embolization from CT pulmonary angiography (CTPA) is necessary to diagnose PTTM. Therefore, for patients with cancer manifesting with severe PH but no arterial embolization from CTPA, CT-guided lung puncture biopsy or bronchoscopy lung biopsy is recommended to rule out the possibility of a missed diagnosis of PTTM. The condition of our patient with both hepatocellular carcinoma (HCC) and gastric signet-ring cell carcinoma who developed PTTM deteriorated faster than that of patients with gastric carcinoma only. Furthermore, we have reviewed 19 reported cases (4-19). We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-3107).
Case presentation
A 62-year-old Chinese man was diagnosed with HCC and liver cirrhosis caused by hepatitis B in 2011. He had undergone radiofrequency ablation. In December 2018, he visited our hospital due to HCC recurrence and was treated with transhepatic arterial chemotherapy and embolization (TACE). However, he developed hepatic encephalopathy, and liver transplantation was performed in February 2019. Postoperative pathology revealed the following: middle caudate lobe differentiated HCC, nodular type, 1-cm diameter, liver capsule invasion, microvascular invasion (MVI) grade = M0, and nerve invasion (−). A necrotic nodule measuring 4 cm in diameter was observed in the right liver. Subsequently, he was regularly treated with tegafur, gimeracil, and oteracil potassium; his condition remained stable.
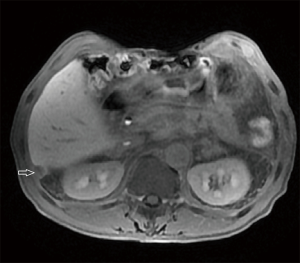
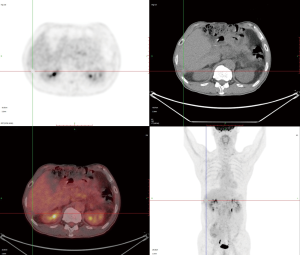
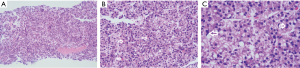
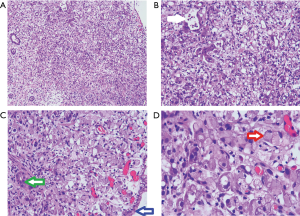
In March 2020, he had a follow-up visit. His hepatobiliary contrast magnetic resonance imaging showed a sub-peritoneal nodule at the right posterior margin of the liver (Figure 1). Moreover, positron emission tomography (PET) showed no increased fluorodeoxyglucose (FDG) metabolism in the peritoneal nodules at the right posterior edge of the liver, and part of the small intestine’s FDG metabolism increased, and endoscopy was recommended (Figure 2). A biopsy of the sub-peritoneal nodules at the posterior margin of the liver showed HCC (Figure 3), and radiofrequency ablation was performed. The patient was treated with chemotherapy (oxaliplatin, calcium folinate, and fluorouracil). He had a gastroscope, which unfortunately showed poorly differentiated adenocarcinoma (gastric signet-ring cell carcinoma) (Figure 4).
Before chemotherapy for gastric signet-ring cell carcinoma, he developed sudden onset of severe dyspnea and hemoptysis on May 16, 2020. Blood gas showed pH of 7.44, oxygen saturation (SpO2) of 80.9%, oxygen tension of 44 mmHg, carbon dioxide tension of 30.9 mmHg, bicarbonate level of 22.4 mmol/L, and base excess (BE) of −1.8 mmol/L, without oxygen (Table 1). Coagulation showed that the D-dimer level was significantly increased to 38,720 µg/L (159 times the normal value) and fibrinogen was decreased to 1.72 g/L (72% of normal) with a prothrombin time activity of 13.7 s (1.1 times the normal value), suggestive of DIC. In Table 1, the blood platelet count decreased to 75×109/L (60% of normal), and the white blood cell count was normal with a C-reactive protein level of 96 mg/L (9.6 times the normal value) (Table 1). Biochemistry was generally normal with low serum albumin (32.8 g/L; 82% of normal) (Table 1).
Table 1
| Examination | Results |
|---|---|
| Hematology | |
| WBC | 7.2×109/L |
| Neutro | 77.8% |
| Lymp | 5.7% |
| Eos. | 1.8% |
| Bas. | 1.0% |
| Mon. | 13.7% |
| RBC | 3.94×1012/L |
| Hb | 121 g/L |
| Hct | 35% |
| Plt | 75×109/L |
| Coagulation | |
| PT | 13.7 s |
| INR | 1.1 |
| APTT | 36 s |
| Fibrinogen | 1.72 g/L |
| D-dimer | 38,720 μg/L |
| Biochemistry | |
| TP | 58.6 g/L |
| Alb | 32.8 g/L |
| Cr | 76 μmol/L |
| T-Bil | 12 μmol/L |
| D-Bil | 5 μmol/L |
| AST | 25 U/L |
| ALT | 10 U/L |
| ALP | 416 U/L |
| γ-GTP | 16 U/L |
| ChE | 5,688 U/L |
| Na | 142 mmol/L |
| K | 4.52 mmol/L |
| Cl | 105 mmol/L |
| P | 0.62 mmol/L |
| Ca | 2.21 mmol/L |
| CRP | 96 mg/L |
| TG | 1.16 mmol/L |
| HDL-C | 0.65 mmol/L |
| LDL-C | 3.6 mmol/L |
| Marker | |
| HBsAg | − |
| Anti-HBs | + |
| Anti-HBc | + |
| Anti-HCV | − |
| AFP | 2.0 ng/mL |
| CEA | 7.6 ng/mL |
| CA125 | 26.6 U/mL |
| CA199 | 878.9 U/mL |
| Fer | 7.3 ng/mL |
| Blood gas | 2020.5.16 no O2 |
| SpO2 | 80.9% |
| pH | 7.44 |
| pCO2 | 30.9 mmHg |
| pO2 | 44 mmHg |
| HCO3 | 20.7 mmol/L |
| BE | −1.8 mmol/L |
WBC, white blood cell; AFP, α-fetoprotein; Alb, albumin; ALP, alkaline phosphatase; ALT, glutamic-pyruvic transaminase; APTT, activated partial thromboplastin time; AST, glutamic oxalacetic transaminase; Bas., basophil; BE, base excess; CA125, cancer antigen 125; CA199, cancer antigen 199; CEA, carcinoembryonic antigen; ChE, cholinesterase; Cr, creatinine; CRP, C-reactive protein; D-Bil, direct bilirubin; Eos., eosinophil; Fer, ferritin; Hb, hemoglobin; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; Hct, hematocrit; HDL-C, high-density lipoprotein cholesterol; INR, international normalized ratio; LDL-C, low-density lipoprotein cholesterol; Lymp, lymphocyte; Mon., monocyte; Neutro, neutrophil; Plt, platelet; PT, prothrombin time; RBC, red blood cell; SpO2, percutaneous oxygen saturation; T-Bil, total bilirubin; TG, triglyceride; TP, total protein; γ-GTP, γ-glutamyl transpeptidase.
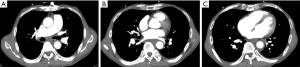
Chest radiography showed a new frosted glass-like shadow on May 16, 2020, compared to the image on May 11, 2020 (Figure 5), while CTPA showed no arterial embolization (Figure 6). Cardiac ultrasound showed severe tricuspid regurgitation, which suggested high pulmonary arterial hypertension. Anticoagulant therapy and antibiotics were administered, but his condition worsened, and he died 3 days (May 18, 2020) after oxygen supplementation.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Final diagnosis
The diagnosis was PTTM, likely caused by tumor cell embolization of the HCC and gastric signet-ring cell carcinoma after multidisciplinary expert discussion, which led to rapid respiratory failure.
Treatment
Since PTTM was considered, we used enoxaparin 40 mg subcutaneous injection once a day, oxygen therapy, and cephalosporin for possible secondary infection. We administered 40 mg methylprednisolone intravenous injection once a day to reduce the inflammatory reaction in the lung.
Outcome and follow-up
The patient’s condition worsened rapidly and died 3 days after oxygen supplementation.
Discussion
PTTM is a rare form of pulmonary arterial tumor embolism. Fibrocellular intimal proliferation of the small pulmonary arteries and arterioles in patients with metastatic carcinoma is among its histological characteristics and is associated with the development of clinical signs of PH that result in acute or subacute cor pulmonale and subacute respiratory failure (1).
In a report by Uruga et al. (3), who surveyed the findings of 2,215 consecutive autopsy cases of carcinoma, 30 patients (1.4%) were diagnosed with PTTM for sure. The most common symptom was progressive dyspnea. Hypercoagulability of blood was observed in all measured cases (n=21). The chest CT findings (n=6) included small nodules, ground-glass opacity, a tree-in-bud appearance and consolidation. The median survival time was nine days after the initiation of oxygen supplementation. The most common original site was the stomach (n=18; 60%), and the most common pathological type was adenocarcinoma (28/30, 93.3%). PTTM caused by multiple tumors, such as HCC and gastric signet-ring cell carcinoma, has been rarely reported. Since the most common original site of PTTM was the stomach, we queried the term ‘‘PTTM’’ and “gastric” on Pubmed.gov database in June of 2020 and received 48 results. We have reviewed 19 reported cases of PTTM caused by gastric carcinoma, except in our case (4-19) (Table 2).
Table 2
| Case | Ref. | Age (year) | Gender | Symptom | Respiratory failure | Pulmonary arterial hypertension |
Diagnosis | Treatment | Outcome | Prognosis (days) | Image of lung | Pathology of lung | Pathology of stomach |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cui et al. (4) | 38 | F | Dyspnea, hemoptysis, chest pain | + | 71 mmHg | Immunocytochemistry of the pleural effusions | Tegafur, gimeracil and oteracil potassium, apatinib, oxygen | Death | 12 | Multiple patchy infiltrating shadows, moderate right pleural effusion | N/A | Poorly differentiated adenocarcinoma |
| 2 | Kubota et al. (5) | 56 | F | Dyspnea | + | 47 mmHg | N/A | Oxygen, dobutamine and bosentan, imatinib | Death | 210 | No parenchymal lesion, enlargement of the para-aortic lymph nodes | N/A | Gastric signet-ring cell carcinoma |
| 3 | Mandaliya et al. (6) | 41 | F | Dyspnea, cough | + | N/A | N/A | Anticoagulation with heparin and intravenous corticosteroids | Death | 7 | Nodular opacities with tree in bud appearance | N/A | Gastric signet-ring cell carcinoma |
| 4 | Tateishi et al. (7) | 75 | M | Cough and weight loss | + | 38 mmHg | Autopsy | Chemotherapy, oxygen | Death | 35 | Reticulonodular opacities, interlobular septal thickening | Widespread tumor emboli in the peripheral small pulmonary arteries | Gastric signet-ring cell carcinoma |
| 5 | Belhassine et al. (8) | 53 | F | Cough, dyspnea, occasional night sweats and fever | + | 39 mmHg | Transbronchial biopsies | Oxygen | N/A | N/A | Numerous bilateral small nodules, ground glass opacities with enlarged interlobular septa, pleural thickening with a small pleural effusion | N/A | Gastric adenocarcinoma |
| 6 | Yokomine et al. (9) | 64 | M | No remarkable symptoms of respiration | – | N/A | Autopsy | N/A | N/A | N/A | Negative | Small arteries and arterioles were stenotic or occluded by fibrocellular intimal proliferation and thromboemboli | Poorly differentiated gastric carcinoma |
| 7 | Ho et al. (10) | 41 | F | Dyspnea | + | N/A | Autopsy | Oxygen and co-amoxiclav and clarithromycin | Death | 8 | Diffuse tiny centrilobular soft tissue nodules forming a tree-in-bud appearance | Widespread tumor emboli with associated thromboemboli in the subsegmental branches of the pulmonary arterial tree | Poorly differentiated gastric adenocarcinoma with signet-ring cell |
| 8 | Kuwabara et al. (11) | 43 | M | Dyspnea, dry cough, rhinorrhea | + | N/A | Autopsy | Antibiotic administration, oxygen | Death | 5 | Diffuse centrolobular tiny nodules and thickening of both bronchovascular bundles and interlobular septa in lungs | Widespread fibrin thrombi and fibrocellular and fibromuscular intimal proliferation with or without gastric cancer cells | Poorly differentiated gastric adenocarcinoma |
| 9 | Endicott-Yazdani et al. (12) | 52 | F | Dyspnea, cough, nausea, vomiting, and vague abdominal pain | + | 37 mmHg | Autopsy | Intravenous epoprostenol, oxygen | Death | 6 | N/A | Arteriolar thickening consisting of medial hypertrophy and intimal fi broplasia in lungs, organizing thrombi with recanalization and anaplastic tumor cells were observed in small pulmonary vessels | Poorly differentiated gastric adenocarcinoma |
| 10 | Rudkovskaia et al. (13) | 49 | M | Dyspnea, cough | + | 70 mmHg | Autopsy | Prednisone, oxygen | Death | N/A | Multiple nodules in both lungs, with surrounding ground-glass opacities and evidence of tree-in-bud pattern | Diffuse dissemination of tumor cells in the lymphatic channels and small pulmonary vessels | Gastro-esophageal junction adenocarcinoma |
| 11 | Chinen et al. (14) | 62 | M | No remarkable symptoms of respiration | – | N/A | Autopsy | Mechanical ventilation | Death | 9 | N/A | Multiple tumor emboli were apparent in the small arteries and arterioles, together with fibrin thrombi, with or without tumor emboli | Gastric poorly and moderately differentiated tubular adenocarcinoma |
| 12 | Sato et al. (15) | 50 | M | Dyspnea | + | N/A | Autopsy | Chemotherapy, oxygen | Death | 2 | A mild expansion of the right lower mediastinal shadow indicating right ventricular enlargement and no cancer metastasis in the lung fields or cardiomegaly | Most of the small muscular arteries and arterioles were stenosed or occluded by fibrocellular intimal proliferation with or without cancer cells in lungs | Poorly differentiated gastric adenocarcinoma |
| 13 | McAnearney et al. (16) | 41 | M | Dyspnea and cough | + | N/A | N/A | Antibiotic, steroid, oxygen | Death | 24 | Reticular nodular interstitial change with alveolar infiltrates and thickening of the secondary pulmonary lobule | N/A | Gastric signet-ring cell carcinoma |
| 14 | Seol et al. (17) | 46 | F | Dyspnea | + | 52 mmHg | N/A | Oxygen | Death | 1 | No evidence of pulmonary emboli | N/A | Gastric cancer |
| 15 | Seol et al. (17) | 48 | M | Dyspnea | + | 70 mmHg | N/A | Oxygen, vasoactive drug | Death | 1 | No evidence of pulmonary emboli | N/A | Gastric cancer |
| 16 | Keenan et al. (18) | 40 | M | Dyspnea, cough, weight loss, chest pain | + | 60 mmHg | Autopsy | Oxygen, anticoagulation with heparin | Death | 11 | No evidence of pulmonary emboli | Intimal fibrosis of the arteries, focal fresh emboli of fibrin mixed with tumor cells and areas of recanalization in lungs | Poorly differentiated gastric adenocarcinoma |
| 17 | Gainza et al. (19) | 36 | M | Weakness, dark urine | + | N/A | Autopsy | Oxygen, plasma exchange | Death | 2 | Bilateral interstitial pulmonary infiltrates | Carcinoma emboli in perivascular lymphatic vessels | Gastric signet-ring cell carcinoma |
| 18 | Gainza et al. (19) | 34 | M | Cough, back pain | + | N/A | N/A | Oxygen, plasma exchange | Death | 12 | Bilateral interstitial pulmonary infiltrates, infracentrimetric mediastinal and retroperitoneal lymphadenopathies | N/A | Gastric signet-ring cell carcinoma |
| 19 | Gainza et al. (19) | 24 | F | Back pain, nausea, vomiting | + | N/A | Autopsy | Oxygen | Death | 2 | Bilateral interstitial pulmonary infiltrates | Blood vessels with eccentric intimal fibrosis, intravascular fibrin thrombi, and recanalization and intraluminal emboli of neoplastic cells | Poorly differentiated gastric adenocarcinoma |
| Our case | N/A | 62 | M | Dyspnea, hemoptysis | + | N/A | N/A | Oxygen, anticoagulation, antibiotic, steroid | Death | 3 | New frosted glass–like shadow around terminal pulmonary vessel, no arterial embolization | N/A | Hepatocellular carcinoma and gastric signet-ring cell carcinoma |
Prognosis: survival after supplemental oxygen (days). N/A, data not available; PTTM, pulmonary tumor thrombotic microangiopathy.
According to our summary, the median survival time after oxygen supplementation was 18 days in PTTM patients caused by gastric carcinoma, which is longer than 9 days, as reported by Uruga et al. (3). However, our patient with both HCC and gastric signet-ring cell carcinoma showed a poorer prognosis, with a survival time of only 3 days (very poor compared to the average level). As tumor embolism, multiple microthrombi, and intimal myofibroblast proliferation in the pulmonary arteries and arterioles are among its histopathological characteristics (1,2), multiple tumors might increase the tumor burden, resulting in more tumor embolism, resulting in faster progression.
Respiratory discomfort is the main symptom of PTTM. Of the 19 patients, 15 (79%) displayed symptoms of respiratory discomfort. In addition, 13 had dyspnea, 9 had cough, 2 had chest pain, 1 had hemoptysis, and 2 had no respiratory difficulties. Moreover, 17 (89%) manifested respiratory failure, leading to pulmonary arterial hypertension and right-sided heart failure. Nearly half of these cases showed severe pulmonary arterial hypertension, which was 54 mmHg on average (9 cases). The clinical manifestations of PTTM depend on the severity of the PH. The degree of pulmonary arteries stenosis is diverse from each other, and it is suggested that PH in PTTM occurs in selected cases with widespread pulmonary lesions with severe smaller arteries stenosis (20).
Abnormal pulmonary imaging was reported in 12 (63%) cases with the appearance of septal thickening, ground-glass opacities, small nodules, pleural effusion, and a tree-in-bud pattern. Ground-glass opacification may reflect the tumor infiltration of the alveolar septae (21). Nodules are generally seen in a centrilobular distribution and are most likely to result from the hematogenous spread of malignancy through the pulmonary arterioles. The histologic correlation of septal thickening is known as the engorgement of lymphatic channels (22). Therefore, there are no specific imaging findings directly suggesting tumor embolism or pulmonary embolism. Our case shows a new frosted glass-like shadow around the terminal pulmonary vessel in a short time with no arterial embolization, which is highly considered in the presence of both HCC and gastric signet-ring cell carcinoma.
All patients with respiratory failure were administered oxygen, and additional treatments included anticoagulation in three cases, antibiotics in three cases, chemotherapy in three cases, steroids in two cases, plasma exchange in two cases, and imatinib in one case. No effective therapeutic options have been established for PTTM. Under the treatment, the survival time was longer than expected with the natural progression of PTTM. Among the important factors leading to a longer survival may be that the treatment regimen results in improvement in pulmonary pressures and/or cardiac output, in addition to treatment of the malignancy (23). The shortest period between the commencement of treatment for respiratory failure and death was 1 day, the longest was 210 days, and the average was 18 days. Case 2, who was treated with imatinib, had the longest survival time of 210 days. Imatinib, a platelet-derived growth factor (PDGF) receptor inhibitor, may play a role in reducing the vascular remodeling that promulgates PH. Although it has shown promise by prolonging survival time in some cases, its role may be limited to various factors such as the type of primary malignancy; any further use of imatinib should be studied more extensively (24). Bevacizumab, a vascular endothelial growth factor (VEGF) receptor inhibitor, has also been used in one case in conjunction with S-1 chemotherapy and imatinib, where the patient experienced a survival of 12 months after a diagnosis of PTTM (25).
Conclusions
PTTM should be considered in patients with cancer who progress to rapid respiratory failure and PH, especially in patients with multiple tumors whose condition is more likely to progress quickly.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-3107
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-3107). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer 1990;66:587-92. [Crossref] [PubMed]
- Chinen K, Tokuda Y, Fujiwara M, et al. Pulmonary tumor thrombotic microangiopathy in patients with gastric carcinoma: an analysis of 6 autopsy cases and review of the literature. Pathol Res Pract 2010;206:682-9. [Crossref] [PubMed]
- Uruga H, Fujii T, Kurosaki A, et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med 2013;52:1317-23. [Crossref] [PubMed]
- Cui N, Wang L, Zhao J. Pulmonary tumour thrombotic microangiopathy presented as gastric signet ring cell carcinoma: a case report. J Int Med Res 2020;48:300060520910884. [Crossref] [PubMed]
- Kubota K, Shinozaki T, Imai Y, et al. Imatinib dramatically alleviates pulmonary tumour thrombotic microangiopathy induced by gastric cancer. BMJ Case Rep 2017;2017:bcr2017221032. [Crossref] [PubMed]
- Mandaliya R, Farhat S, Uprety D, et al. Occult gastric cancer presenting as hypoxia from pulmonary tumor thrombotic microangiopathy. J Gastric Cancer 2014;14:142-6. [Crossref] [PubMed]
- Tateishi A, Nakashima K, Hoshi K, et al. Pulmonary tumor thrombotic microangiopathy mimicking inhalation lung injury. Intern Med 2019;58:1311-4. [Crossref] [PubMed]
- Belhassine M, Papakrivopoulou E, Venet C, et al. Gastric adenocarcinoma revealed by atypical pulmonary lymphangitic carcinomatosis. J Gastrointest Oncol 2018;9:1207-12. [Crossref] [PubMed]
- Yokomine T, Hirakawa H, Ozawa E, et al. Pulmonary thrombotic microangiopathy caused by gastric carcinoma. J Clin Pathol 2010;63:367-9. [Crossref] [PubMed]
- Ho AL, Szulakowski P, Mohamid WH. The diagnostic challenge of pulmonary tumour thrombotic microangiopathy as a presentation for metastatic gastric cancer: a case report and review of the literature. BMC Cancer 2015;15:450. Erratum in: BMC Cancer 2015 Aug 26;15:600. doi: 10.1186/s12885-015-1601-6. [Crossref] [PubMed]
- Kuwabara H, Yoshida S, Takasu T, et al. Pulmonary tumor thrombotic microangiopathy caused by gastric cancer. Ann Thorac Med 2012;7:168-9. [Crossref] [PubMed]
- Endicott-Yazdani T, Ghazi A, Armstrong D, et al. Fatal pulmonary tumor thrombotic microangiopathy caused by undiagnosed metastatic gastric adenocarcinoma. Proc (Bayl Univ Med Cent) 2015;28:482-3. [Crossref] [PubMed]
- Rudkovskaia AA, Lo YC, Brady V, et al. A 49-year-old man with subacute respiratory failure and interstitial lung opacities. Am J Case Rep 2017;18:941-4. [Crossref] [PubMed]
- Chinen K, Kazumoto T, Ohkura Y, et al. Pulmonary tumor thrombotic microangiopathy caused by a gastric carcinoma expressing vascular endothelial growth factor and tissue factor. Pathol Int 2005;55:27-31. [Crossref] [PubMed]
- Sato Y, Marutsuka K, Asada Y, et al. Pulmonary tumor thrombotic microangiopathy. Pathol Int 1995;45:436-40. [Crossref] [PubMed]
- McAnearney S, Drain M. A case of pulmonary tumour thrombotic microangiopathy. Respir Med Case Rep 2015;16:7-10. [Crossref] [PubMed]
- Seol SH, Park BM, Jin HY, et al. Fatal acute right heart failure in gastric cancer patients. Heart Views 2013;14:179-81. [Crossref] [PubMed]
- Keenan NG, Nicholson AG, Oldershaw PJ. Fatal acute pulmonary hypertension caused by pulmonary tumour thrombotic microangiopathy. Int J Cardiol 2008;124:e11-3. [Crossref] [PubMed]
- Gainza E, Fernandez S, Martinez D, et al. Pulmonary tumor thrombotic microangiopathy: report of 3 cases and review of the literature. Medicine (Baltimore) 2014;93:359-63. [Crossref] [PubMed]
- Okubo Y, Wakayama M, Kitahara K, et al. Pulmonary tumor thrombotic microangiopathy induced by gastric carcinoma: morphometric and immunohistochemical analysis of six autopsy cases. Diagn Pathol 2011;6:27. [Crossref] [PubMed]
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997;169:355-67. [Crossref] [PubMed]
- Godbole R, Saggar R, Zider A, et al. Insights on pulmonary tumor thrombotic microangiopathy: a seven-patient case series. Pulm Circ 2017;7:813-20. [Crossref] [PubMed]
- Godbole RH, Saggar R, Kamangar N. Pulmonary tumor thrombotic microangiopathy: a systematic review. Pulm Circ 2019;9:2045894019851000. [Crossref] [PubMed]
- Price LC, Wells AU, Wort SJ. Pulmonary tumour thrombotic microangiopathy. Curr Opin Pulm Med 2016;22:421-8. [Crossref] [PubMed]
- Higo K, Kubota K, Takeda A, et al. Successful antemortem diagnosis and treatment of pulmonary tumor thrombotic microangiopathy. Intern Med 2014;53:2595-9. [Crossref] [PubMed]



