
Predicting factors of tumor progression in adult patients with low-grade glioma within five years after surgery
Introduction
Glioma is the most common primary intracranial tumor, which originates from glial cells in the brain. The WHO divides gliomas into grades I to IV, with grades I and II as low grades, and grades III and IV as high grades, while grade I is often difficult to diagnose early in clinical practice. Over 14,000 new cases are diagnosed in the United States each year. The annual incidence rate of glioma in China is (3.0–6.4)/100,000, the annual incidence rate of malignant glioma reaching 5.8/100,000, and the number of new patients per year can reach 7,000–10,000 cases. Glioblastoma (GBM) is WHO grade IV, accounting for about 50% of primary malignant central nervous system gliomas. In the past 20 years, the incidence of glioma has increased slightly, especially among the elderly. It may be because of the advancement of imaging diagnostics, improved living standards, and increased patient awareness and demand for diagnosis and treatment, but the overall trend is increasing in younger patients. The incidence of malignant glioma in men is 40% higher than that in women, and whites are twice black people. The median age of patients diagnosed with GBM was 64 years, and anaplastic glioma was 45 years. Glioma is a malignant tumor with the worst prognosis, whose 5-year mortality rate is second only to pancreatic cancer and lung cancer among systemic tumors. The pathogenesis is still unclear, and the more certain risk factor is high-dose ionizing radiation exposure.
Low-grade gliomas include gliomas of astrocyte origin and oligodendrocyte origin, which often occur on the screen and accounts for 30% of all gliomas (1). Compared with high-grade gliomas, low-grade gliomas tend to occur in young people between the ages of 30 and 40, who usually have the characteristics of well-differentiated, slow growth, and low invasiveness. Therefore, patients with low-grade gliomas have a longer survival time. At present, the ideal treatment method makes the median survival time (MST) of these patients reach seven years (average survival time is 5 to 14 years). Timely diagnosis and surgery are the main treatment options for low-grade gliomas, and the prognosis is relatively good. However, many patients relapse or continue to progress after treatment, leading to adverse outcomes.
Previous studies mostly focused on all patients with gliomas, without distinguishing the WHO grades. Some studies did not take the factor of surgery into account, and some studies aimed at high-grade (WHO grades III–IV) patients. In particular, there are few studies on the follow-up outcomes of patients with WHO grade II glioma after surgery (1-4). The innovation of our study is to retrospectively analyze the case data of our medical center to explore the factors related to the prognosis of patients with WHO grade II glioma within 5 years after surgery including: age >50 years, incomplete tumor resection, tumor diameter >5 cm and long-term preoperative use of statins, etc. Univariate and multivariate analysis were used to analyze the related factors of tumor progression during 5-year follow-up after surgery, receiver operating curve (ROC) analysis was used to quantitatively analyze the optimal cut-off value of distinguishing the high and low risks of treatment and Kaplan-Meier survival curve analysis was used to analyze the predictive value of risk factors for 5-year follow-up results after surgery. It has important clinical significance for identifying high-risk patients as early as possible. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-21-589).
Methods
Materials
A retrospective analysis of patients who received their first surgery for glioma in our hospital from February 2011 to May 2015. Inclusion criteria: (I) age over 18 years; (II) definite diagnosis of primary glioma; (III) WHO grade II; (IV) first surgical resection of the tumor. Exclusion criteria: (I) combined with other brain tumors or extra-brain malignant tumors; (II) combined with heart failure, renal failure, liver failure; (III) combined with connective tissue disease; (IV) long-term use of glucocorticoids for other diseases before or after surgery; (V) long-term use of immunosuppressive agents for other diseases before or after surgery. According to the inclusion and exclusion criteria, 287 patients were included in the final analysis. All procedures involving human participants in this study follow the Declaration of Helsinki (revised in 2013). Handan Central Hospital approved this study (approval number: 20200603), and the informed consent of all patients was obtained.
Study methods
All patients underwent surgical resection, and the operations were all planned and completed by the same glioma surgery team. A total of 121 patients (42.2%) underwent extended tumor resection or total resection, 166 patients (57.8%) received partial resection, of which 131 (45.6%) patients underwent subtotal resection, and 35 patients (12.2%) received partial resection. After surgery, 47 patients (16.4%) received radiotherapy, 42 patients (14.6%) received chemotherapy, 85 patients (29.6%) received both radiotherapy and chemotherapy, and 113 patients received neither radiotherapy or chemotherapy (39.4%).
Follow-up
All patients were followed up regularly after discharge from the hospital, one month, three months, six months, and 12 months after surgery, and once a year after that. During the period, follow-up visits are required at any time according to the condition. Follow-up content includes brain MRI review, EEG review, blood test indicators, including white blood cell count, red blood cell count, platelet count, neutrophil count, lymphocyte count, hemoglobin concentration, alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, serum urea nitrogen concentration, serum creatinine concentration, serum uric acid concentration, electrolyte level. Adverse events include syncope, seizures, sudden death, cerebral hemorrhage.
Observation index
After the patients were enrolled, the baseline data before the first operation were collected. The demographic data included age, gender, family income, etc. The medical history data included underlying diseases, smoking, drinking, blood pressure, heart rate, weight, height, and commonly used blood test indicators (including white blood cell count, red blood cell count, platelet count, neutrophil count, lymphocyte count, hemoglobin concentration, alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, serum urea nitrogen concentration, serum creatinine concentration, serum uric acid concentration, electrolyte level), imaging data (including the imaging data of the first diagnosis of glioma and the most recent imaging data before surgery, including the location and size of the tumor). Tumor progression is defined as: imaging shows there are recurring tumor signals at the original surgical site, and the original tumor tissue left behind is larger than the results of the postoperative review, the postoperative imaging-confirmed surgery-related complications, and the resulting deaths, and other tumor-related deaths.
Statistical processing
Statistical processing was performed with SPSS 22.0 statistical software. Quantitative data were tested for normal homogeneity, the mean ± standard deviation represented the data conforming to the normal distribution, and the student t-test was used for comparison between groups; the median represented those that did not comply with the normal distribution, and the rank-sum test represented the comparison between groups; numerical values and percentages expressed qualitative data, and comparisons between groups were by x2 test or Fisher’s exact test. The relationship between single factor and multiple factors analysis and the prognosis of patients during follow-up. The receiver operation curve (ROC) was used to analyze the greatest quantitative treatment boundary value to distinguish high and low risks. Kaplan-Meier survival curves were used to analyze the influence of distinct factors on the long-term prognosis of patients. P<0.05 showed the difference was statistically significant.
Results
Results of 5-year follow-up
All patients were followed up for more than five years. During the 5-year follow-up period, 122 cases (42.5%) had not progression (progression-free group, PFG), 165 cases (57.5%) had progression (progression group, PG), of which 97 cases (33.8%) had reoperation, 41 cases (14.3%) non-surgical treatment, 34 cases (11.8%) all-cause deaths, and 27 cases (9.4%) tumor-related deaths, as shown in Table 1.
Table 1
| Adverse events | Number | Percentage |
|---|---|---|
| Progression (n, %) | 165 | 57.5 |
| Repeated surgery (n, %) | 97 | 33.8 |
| Non-surgery treatment (n, %) | 41 | 14.3 |
| Tumor-related death (n, %) | 27 | 9.4 |
| All-cause death (n, %) | 34 | 11.8 |
| Symptom aggravation (n, %) | 13 | 4.5 |
Baseline characteristics comparison of patients with and without tumor progression
Among the 287 patients, 154 (53.7%) were male, and 133 (46.3%) were female. The age ranged from 18 to 71 years, with an average of 40.2±13.8 years old. Among them, 162 cases (56.4%) were under 40 years old, 107 cases (37.3%) were 40 to 65 years old, and 18 cases were over 65 years old (6.3%). According to whether they have progression during follow-up, they are divided into progression groups (PG) and progression-free groups (PFG). The baseline data comparison between the two groups was shown in Tables 2 and 3. The results showed that there were statistical differences in multiple indicators between the two groups of baseline data, including age, the proportion of smoking patients, the proportion of drinking patients, the neutrophil/lymphocyte ratio in the blood test results, tumor diameter, and operation method, age, and proportion of statins taken before surgery.
Table 2
| Characteristic | PG (n=165) | PFG (n=122) | t/X2 value | P value |
|---|---|---|---|---|
| Age (years) | 42.7±11.2 | 36.8±9.6 | 4.683 | <0.01 |
| Male (n, %) | 84 (50.9) | 70 (57.4) | 1.180 | 0.277 |
| BMI (kg/m2) | 23.5±3.8 | 22.6±3.5 | 2.051 | 0.041 |
| Smoking (n, %) | 62 (37.6) | 32 (26.2) | 4.100 | 0.043 |
| Alcohol (n, %) | 67 (40.6) | 35 (28.7) | 4.348 | 0.037 |
| WBC (×109/L) | 6.8±2.4 | 6.4±2.4 | 1.396 | 0.164 |
| NEU (×109/L) | 3.9±1.6 | 3.8±1.5 | 0.537 | 0.591 |
| LYM (×109/L) | 2.1±0.6 | 2.2±0.6 | 1.538 | 0.125 |
| RBC (×1012/L) | 4.8±1.0 | 4.9±1.1 | 0.802 | 0.423 |
| Hb (g/L) | 142.6±14.7 | 144.5±15.4 | 1.061 | 0.290 |
| PLT (×109/L) | 195.9±32.8 | 201.3±34.6 | 1.347 | 0.179 |
| NLR | 1.86±0.52 | 1.73±0.49 | 2.145 | 0.033 |
| PLR | 93.3±21.4 | 91.5±22.8 | 0.685 | 0.494 |
| Cr (μmol/L) | 68.5±19.4 | 71.3±21.7 | 1.149 | 0.252 |
| UA (μmol/L) | 268.6±51.5 | 273.2±58.1 | 0.708 | 0.479 |
| ALT (U/L) | 26.9±9.8 | 27.4±10.2 | 0.420 | 0.675 |
| AST (U/L) | 22.5±5.3 | 21.8±5.2 | 1.115 | 0.266 |
| TBIL (μmol/L) | 12.7±4.9 | 13.3±5.3 | 0.990 | 0.323 |
| DBIL (μmol/L) | 5.9±2.1 | 6.1±2.6 | 0.720 | 0.472 |
| BUN (mmol/L) | 7.3±2.6 | 6.9±2.8 | 1.247 | 0.214 |
| K (mmol/L) | 4.52±0.33 | 4.58±0.34 | 1.503 | 0.134 |
| Na (mmol/L) | 142.8±10.7 | 143.6±11.2 | 0.614 | 0.540 |
| Cl (mmol/L) | 107.1±12.9 | 108.2±13.6 | 0.698 | 0.486 |
| Ca2+ (mmol/L) | 2.1±0.4 | 2.2±0.5 | 1.881 | 0.061 |
| Hypertension (n, %) | 16 (9.7) | 15 (12.3) | 0.491 | 0.483 |
| Hyperlipidemia (n, %) | 19 (11.5) | 16 (13.1) | 0.167 | 0.682 |
| Diabetes (n, %) | 6 (3.6) | 4 (3.3) | 0.026 | 0.871 |
| CAD (n, %) | 8 (4.8) | 6 (4.9) | 0.001 | 0.978 |
| COPD (n, %) | 2 (1.2) | 2 (1.6) | 0.042 | 0.838 |
| Aspirin (n, %) | 27 (16.4) | 25 (20.5) | 0.806 | 0.369 |
| Statins (n, %) | 11 (6.7) | 23 (18.9) | 9.973 | 0.002 |
| ADDs (n, %) | 3 (1.8) | 4 (3.3) | 0.628 | 0.428 |
PG, progression group; PFG, progression-free group; BMI, body mass index; WBC, white blood cell; NEU, neutrophil; LYM, lymphocyte; RBC, red blood cell; Hb, hemoglobin; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; Cr, creatine; UA, uric acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; CAD, coronary heart disease; COPD, chronic obstructive pulmonary disease; ADD, anti-diabetic drugs.
Table 3
| Characteristics | Progression (n=165) | Non-progression (n=122) | t/X2 value | P value |
|---|---|---|---|---|
| Location | 1.411 | 0.703 | ||
| Supracerebral lobe (n=219) | 127 (77.0) | 92 (75.4) | ||
| Supracerebral midline (n=34) | 18 (10.9) | 16 (13.1) | ||
| Cerebellum (n=29) | 16 (9.7) | 13 (10.7) | ||
| Brain stem (n=5) | 4 (2.4) | 1 (0.8) | ||
| Diameter (mm) | 32.7±9.3 | 29.4±8.8 | 3.040 | 0.003 |
| Classification | 1.029 | 0.599 | ||
| Oligodendroglioma | 27 (16.4) | 20 (16.4) | ||
| Astrocytoma | 118 (71.5) | 87 (71.3) | ||
| Oligoastrocytoma | 20 (12.1) | 15 (112.3) | ||
| Operation | 6.525 | 0.011 | ||
| Partial resection | 106 (64.2) | 60 (49.2) | ||
| Total/enlarged resection | 59 (35.8) | 62 (50.8) | ||
| Radiotherapy | 0.182 | 0.670 | ||
| Yes | 84 (50.9) | 59 (48.4) | ||
| No | 81 (49.1) | 63 (51.6) | ||
| Chemotherapy | 0.038 | 0.846 | ||
| Yes | 79 (47.9) | 57 (46.7) | ||
| No | 86 (52.1) | 65 (53.3) |
Factors associated with tumor progression within 5-year follow-up
After further univariate and multivariate analysis, the multivariate analysis showed that age older than 50 (OR =1.42, 95% CI: 1.02–3.33, P=0.013), partial resection of tumor (OR =1.86, 95% CI: 1.13–3.02, P=0.027), tumor diameter larger than 5 cm (OR =1.85, 95% CI: 1.18–4.21, P=0.022), and long-term statins treatment before surgery (OR =0.36, 95% CI: 0.15–0.84, P=0.036) and so on, were closely associated with tumor progression within 5-year follow-up, as shown in Table 4.
Table 4
| Factors | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age >50 years | 1.33 | 1.10–3.01 | 0.032 | 1.42 | 1.02–3.33 | 0.013 | |
| Partial resection | 1.86 | 1.15–2.98 | 0.011 | 1.72 | 1.13–3.02 | 0.027 | |
| Diameter >5 cm | 1.71 | 1.12–3.93 | 0.026 | 1.85 | 1.18–4.21 | 0.022 | |
| Long-term statins treatment | 0.31 | 0.15–0.64 | 0.002 | 0.36 | 0.15–0.84 | 0.036 | |
Effects of factors on prognosis Kaplan-Meier
The Kaplan-Meier survival curve showed patients aged older than 50, partial resection of the tumor, a tumor diameter larger than 5 cm, whose 5-year follow-up results were poor, and long-term statins treatment before surgery better prognosis within the 5-year follow-up, as shown in Figure 1.
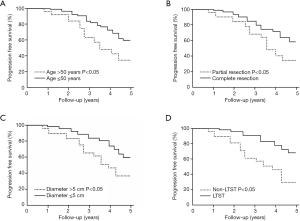
Discussion
This study retrospectively analyzed the case data of our medical center and found that the prognostic factors of patients with WHO grade II glioma within five years after surgery include: patients aged older than 50, partial resection of the tumor, tumor diameter larger than 5 cm, and long-term statins treatment before surgery, among which three factors including patients aged older than 50, partial resection of the tumor, tumor diameter larger than 5 cm increase the risk of tumor progression within five years after surgery, while long-term statin treatment before surgery can reduce the risk of tumor progression within five years after surgery.
WHO grade II glioma usually occurs between 20–40 years old. The clinical symptoms vary by location and tumor size by a considerable extent, related to the tumor’s oppressive effect. The most common symptom is seizures, which can occur at a rate of 60% to 80%. Other common symptoms include headaches, cognitive difficulties, behavioral changes, weakness in movement, or changes in sensation. Seizures are often controlled with antiepileptic drugs. Symptoms usually abate after removal of the tumor in most patients. A retrospective study by Jakola et al. showed that early enlarged resection of tumors could improve patients’ prognosis with WHO grade II glioma (2). If the resection is delayed or unresectable, the tumor growth should be closely monitored through imaging examinations within the first six months of diagnosis. WHO grade II gliomas include astrocytoma, oligodendroglioma, etc. (3). Gliomas in this study accounted for about 10% to 20% of primary brain tumors and affected young people primarily (4).
WHO grade II gliomas are usually well-differentiated and slow-growing tumors, but they are often resistant to treatment. Although these tumors are usually clinically stable for an extended period, only a long interval of imaging can detect their slow progress. If effective surgical treatment is not obtained in time, many WHO grade II gliomas will eventually transform into higher-grade tumors (WHO III-IV) at some point in the disease’s course. Standard treatment includes surgical resection and radiotherapy. Radiotherapy is usually used for patients with symptoms and progressive disease, or patients with a poor prognosis (5). Although the effect of early surgery and the resection scope have not been confirmed in randomized studies, a retrospective study has shown that enlarged resection can improve the prognosis (6). However, in clinical practice, doctors often choose the operation time and operation method according to the patient’s specific conditions, and there are still many patients who will relapse and progress after the operation.
There are many studies on the prognostic factors of gliomas, which mainly include several aspects: (I) basic medical history data, including the results of various preoperative routine blood tests, the patient’s living habits, underlying diseases, and general information (including age, gender); (II) preoperative imaging data, including tumor location, size, relationship with surrounding tissues; (III) tumor pathological data, including pathological classification. Many studies have focused on the relationship between new molecular-level biomarkers, including IDH1 and IDH2 genotypes, related microRNA, protein molecular expression, and glioma. These excellent studies have provided the medical community with various new perspectives on glioma and inspired many clinical diagnosis and treatment ideas. It is presumed it will play a key role in future drug development and early diagnosis. However, some of these studies looked at all patients with gliomas and did not distinguish the WHO classification. Some did not consider the factor of surgery, and some studies targeted high-grade (WHO III-IV) patients. There are few studies on patients’ follow-up outcomes with WHO grade II glioma, and these patients are often groups with satisfactory surgical results. The clinical findings of grade I gliomas are rare, and the results of surgery for WHO III-IV gliomas are often not satisfactory. However, even with WHO grade II glioma, many patients whose postoperative follow-up show poor outcomes. How to screen out high-risk patients from these patients in the early stage for intensive treatment and close monitoring is of considerable clinical significance. Earlier studies have found patients aged >40 years, partial tumor resection, negative IDH1 R132H expression, and positive RTEL1 expression are independent risk factors that affect patients’ progression-free survival with WHO grade II glioma (7). Our research results are partially consistent with it, but our research suggests that a higher age group is closely related to the prognosis; that is, the risk of patients aged over 50 years old is increased. Although the specific age groups are different, the general trend suggests that the older the patients, the worse the postoperative outcome. The older patients should be closely followed up and monitored. We did not conduct relevant genetic testing, nor did we add other unusual clinical blood indicators for analysis. The main reason is although these indicators may have good or better predictive value, they are challenging to be popularized in clinical practice for the time being. And the simple, easy, and inexpensive indicators are needed to supply reference value in clinical practice.
Simultaneously, there have been many studies on the relationship between drug treatment and the prognosis of gliomas in recent years, and statins treatment has received extensive attention. Statins have been proven in many clinical studies to reduce the risk of malignant tumors (8-10) and reduce the risk of tumor-related death (11), but some studies have shown an increased risk of tumors (12,13), and there is no clear relationship between statins and the occurrence or outcome of tumors (14). In a study, Cote et al. analyzed data from three studies: Nurses’ Health Study (NHS) (n=114,419), Nurses’ Health Study II (NHSII) (n=115,813), and Male Health Professionals Follow-up Study (HPFS) (n=50,223), found that compared with patients who had never used statins treatment, patients who had used statins treatment had a significantly higher risk of glioma (HR =1.43, 95% CI: 1.10–1.86), and the longer using statins treatment, the higher the risk of glioma (HR =1.72, 95% CI: 1.21–2.45, for >8 years of use, p-trend =0.003). Further subgroup analysis found that water-soluble statins (including rosuvastatin, pravastatin) were closely related to this risk, while fat-soluble statins (simvastatin, atorvastatin) were not statistically significant (15). However, a meta-analysis combined three earlier studies and found that taking statins can reduce the risk of glioma in the future (OR =0.75, 95% CI: 0.62–0.90, P=0.0016) (16). Some studies have also found no clear relationship between using statins and the risk of glioma. Further subgroup analysis also suggested no clear relationship between using different statins and the risk of glioma (17). The study was included 2,469 patients with glioma and 24,690 cases in the control group. Compared with similar studies, the sample size was larger and had better representativeness. However, unlike many studies, 1,734 (70.2%) patients with glioma in this study were over 50 years old, and half of the patients over 60 years old, and many studies have shown glioma patients have shown patients with glioma are getting younger and younger, especially patients with low-grade gliomas. Many earlier studies did not distinguish the glioma grades, and the developmental biology and prognosis of different grades of gliomas are rare. This study is especially aimed at patients with WHO grade II glioma who have undergone surgery. These patients’ biological behavior with high-grade tumors may be patient-friendly and may have positive responses to drugs. Simultaneously, surgical stimulation also has a certain effect on the biological behavior of tumors (18), and the extra-lipid-lowering effects of statins may have a protective effect on this process. Also, statins may have a certain impact on the local microenvironment of the tumor. Therefore, the results of many studies on statins on the risk of glioma are inconsistent, which may be related to the obvious differences in the included patients. Moreover, most patients in the studies mentioned above are non-Asian, and there may be differences between ethnic groups.
The impact of tumor size and tumor resection area on the 5-year follow-up results of patients with WHO grade II glioma is like other studies (7). The difference lies in the specific numerical range. The larger the tumor is after surgery, the worse the follow-up result will be, and the more thorough the tumor resection, the better the follow-up result will be.
Conclusions
Disadvantages of this study: This study is a single-center retrospective study with small sample size. On the one hand, later research can design and conduct prospective controlled studies for many patients taking statins in China; alternatively, it can conduct multi-center real-world data mining and analysis. However, prospective controlled studies can be designed and conducted for the substantial number of patients taking statins in China; whereas, it can conduct multi-center real-world data mining and analysis in the later research. And simultaneously, relevant experimental research can also be conducted at the cellular level.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-21-589
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-21-589
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-21-589). He Liu is the staff member of the Shenzhen Mindray Bio-Medical Electronic Co. Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures involving human participants in this study follow the Declaration of Helsinki (revised in 2013). Handan Central Hospital approved this study (approval number: 20200603), and the informed consent of all patients was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Le Rhun E, Taillibert S, Chamberlain MC, et al. Current Management of Adult Diffuse Infiltrative Low Grade Gliomas. Curr Neurol Neurosci Rep 2016;16:15. [Crossref] [PubMed]
- Jakola AS, Unsgård G, Myrmel KS, et al. Surgical strategy in grade II astrocytoma: a population-based analysis of survival and morbidity with a strategy of early resection as compared to watchful waiting. Acta Neurochir (Wien) 2013;155:2227-35. [Crossref] [PubMed]
- Bourne TD, Schiff D. Update on molecular findings, management and outcome in low-grade gliomas. Nat Rev Neurol 2010;6:695-701. [Crossref] [PubMed]
- Schiff D, Brown PD, Giannini C. Outcome in adult low-grade glioma The impact of prognostic factors and treatment. Neurology 2007;69:1366-73. [Crossref] [PubMed]
- van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 2005;366:985-90. [Crossref] [PubMed]
- Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 2008;26:1338-45. [Crossref] [PubMed]
- Zhang DX, Qu YM, Zhang MS, et al. Analysis of risk factors influencing the progression-free survival in WHO II grade gliomas. Chin J Neurosurg 2019;35:469-73.
- Ahern TP, Lash TL, Damkier P, et al. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol 2014;15:e461-8. [Crossref] [PubMed]
- Poynter JN, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med 2005;352:2184-92. [Crossref] [PubMed]
- Harshman LC, Wang X, Nakabayashi M, et al. Statin Use at the Time of Initiation of Androgen Deprivation Therapy and Time to Progression in Patients With Hormone-Sensitive Prostate Cancer. JAMA Oncol 2015;1:495-504. [Crossref] [PubMed]
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012;367:1792-802. [Crossref] [PubMed]
- Lin BM, Li WQ, Cho E, et al. Statin use and risk of skin cancer. J Am Acad Dermatol 2018;78:682-93. [Crossref] [PubMed]
- Fujimoto M, Higuchi T, Hosomi K, et al. Association between statin use and cancer: data mining of a spontaneous reporting database and a claims database. Int J Med Sci 2015;12:223-33. [Crossref] [PubMed]
- Emilsson L, García-Albéniz X, Logan RW, et al. Examining Bias in Studies of Statin Treatment and Survival in Patients With Cancer. JAMA Oncol 2018;4:63-70. [Crossref] [PubMed]
- Cote DJ, Rosner BA, Smith-Warner SA, et al. Statin use, hyperlipidemia, and risk of glioma. Eur J Epidemiol 2019;34:997-1011. [Crossref] [PubMed]
- Greenland S. A serious misinterpretation of a consistent inverse association of statin use with glioma across 3 case-control studies. Eur J Epidemiol 2017;32:87-8. [Crossref] [PubMed]
- Seliger C, Meier CR, Becker C, et al. Statin use and risk of glioma: population-based case-control analysis. Eur J Epidemiol 2016;31:947-52. [Crossref] [PubMed]
- Hiller JG, Perry NJ, Poulogiannis G, et al. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 2018;15:205-18. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


