
A case report of endoscopic submucosal dissection for a new subtype of gastric adenoma: mixed fundic and pyloric mucosa type
Introduction
Gastric adenoma is a benign tumor of the stomach, which is more commonly located in the gastric antrum and gastric body. Usually, there is no obvious clinical manifestation (1). However, unlike other common inflammatory polyps, gastric adenoma are associated with a high risk of cancer development. Therefore, once a gastric adenoma is discovered, resection is recommended to prevent it from becoming cancerous. Nowadays, endoscopy has changed from simply providing a diagnosis to providing a combination of diagnosis and treatment. Endoscopic submucosal dissection (ESD) is a new diagnostic and therapeutic method, which is mainly used in the clinical treatment of precancerous lesions and early cancers of the digestive tract. It can be used to remove all the diseased tissues through endoscopy. ESD has the following three advantages: (I) minimally invasive; (II) patients can receive multiple treatments of multiple sites; (III) the doctor may obtain a better pathological specimen for further examination. We report a case of gastric adenoma identified by endoscopy and then treated by ESD. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-197).
Case presentation
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
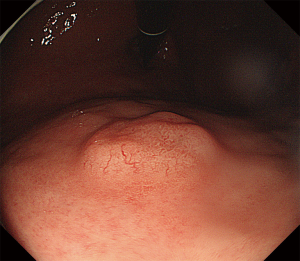
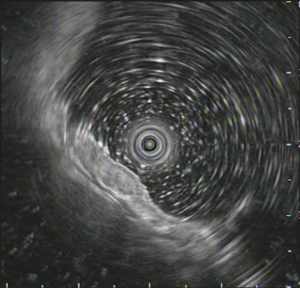
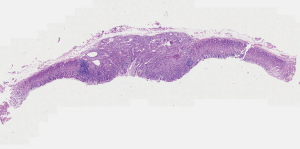
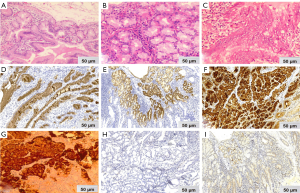
A 48-year-old man with intermittent abdominal bloating for four months to our hospital. Esophagogastroduodenoscopy revealed a 1.2 cm superficial elevated lesion at the anterior wall of the upper gastric body, which had a whitish color and coarse surface (Figure 1). The background mucosa showed the disappearance of collecting venules with O-1 type of atrophic appearance, while the background mucosa covering the lesion was non-atrophic. Detection of Helicobacter by the 13C-urea breath test was positive. Biopsy revealed a low-grade intraepithelial neoplasia (LGIN). Further narrow-band imaging, with magnifying endoscopy revealed a clear demarcation line with an irregular microsurface pattern (Figure 2A,B). Endoscopic ultrasonography revealed that the lesion was mainly restricted to the mucosal layer with suspicious submucosal invasion (Figure 3). Endoscopic submucosal dissection was performed after obtaining a signed informed consent. Histological results revealed gastric adenoma (Figure 4) with mixed fundic and pyloric mucosa type (Figure 5A,B), with high-grade intraepithelial neoplasia (HGIN) (Figure 5C), the horizontal and vertical margins were free, and no submucosal invasion was noted. Immunohistochemical results showed positive expression of MUC5AC(Abcam, ab3649), MUC6(Abcam, ab216017), lysozyme(Abcam, ab91653), pepsinogen I(Abcam, ab50123), and negative expression of MUC2(Abcam, ab231427) and proton pump (H+-K+-ATPase)(Abcam, ab176992) (Figure 5D,E,F,G,H,I). These results suggest that the lesion contained three types of cells: pyloric gland, fundus gland and foveolar epithelium. Helicobacter pylori detection was negative in the lesion. No recurrence was observed during a follow-up period of 9 months.

Discussion
In this case, a mixed fundic and pyloric mucosa type of gastric adenoma was located in the anterior wall of the upper gastric body. The lesion contained three types of cells: pyloric gland, fundus gland and foveolar-epithelium. Precancerous lesions of gastric cancer are classified by the WHO (2019) into low-grade intraepithelial neoplasia and HGIN, and eminence lesions are adenomas (2). Both intestinal and gastric adenomas can be classified according to the direction of differentiation (2). According to the pathological classification, the case reported here does not belong to any of these types. To the best of our knowledge, this is the first case report of gastric adenoma with mixed fundic and pyloric mucosa cell types.
Pyloric gland adenomas (PGAs) are rare neoplasms of the gastrointestinal tract, which mainly occur in elderly women, and arises in patients with autoimmune (metaplastic) atrophic gastritis. The gastric body is the most common location of PGAs, followed by the gastric transition zone, antrum, and cardia (3). PGAs are composed of tightly packed tubular glands lined with cuboidal or columnar cells. Most of the lesions are hemispherical or lobulated and consist of tightly arranged pyloric adenoid cells (4). Immunohistochemical results have showed positive expression of MUC6 and MUC5AC, but in their pure form, they lack expression of MUC2 and CDX2 (5), which can differentiate PGAs from other gastric polyps, such as hyperplastic polyps, and foveolar hyperplasia. PGAs are clinically significant, Vieth et al. (6) found that 30% of gastric PGAs could be transformed into gastric pyloric adenocarcinoma (6).
The recent discovery of MUC genes coding for the core proteins of mucin has identified the phenotypic expression of gastrointestinal neoplasms (7). Most gastric cancers are positive for MUC5AC, MUC6 and MUC2 (8), most adenomas are strongly positive for the intestinal markers MUC2 and CD10, but are negative for the gastric cancer markers MUC5AC and MUC6 (7). The comparison of MUC gene expression in this case and gastric adenoma or cancer is shown in Table 1.
Table 1
| This case | Gastric cancer | Gastric adenoma | |
|---|---|---|---|
| MUC2 | Negative | Positive | Positive |
| MUC5AC | Positive | Positive | Negative |
| MUC6 | Positive | Positive | Negative |
Gastric intraepithelial neoplasia is a precancerous lesion that can be classified as LGIN and HGIN. Approximately 60~85% of patient with HGIN will develop gastric cancer during follow-up (9), and often choose endoscopic treatment. LGIN can also become gastric cancer, however, there are still some divergent views on LGIN treatment. Some scholars recommend endoscopic follow-up for these patients, and some recommend endoscopic treatment (10). However, in recent years, studies (11-13) have shown that there are significant differences between pathological results inferred from preoperative microscopic biopsy and post-ESD, with the incidence of differences ranging from 20% to 44.5%. There are several reasons for this (14): (I) some lesions are difficult to biopsy; (II) the biopsy was inexact; (III) biopsy lesions are small; (IV) the diagnostic ability of the pathologist (14). Therefore, in clinical work, the accuracy of performing the biopsy should be continuously improved. When the size of the LGIN lesion is ≥2 cm, attention should be paid to the possibility of pathological upgrading (15).
Endoscopic therapy can effectively remove early gastric cancer and maintain the quality of life of patients, and has become the preferred treatment for HGIN of gastric tumors (16). The patient was followed up for 9 months after ESD, and there was no recurrence.
In summary, we report a rare case of gastric adenoma that showed the following characteristic clinicopathological findings: (I) he had HP infection but HP negative in the lesion; (II) the background mucosa showed disappearance of collecting venules with O-1 type of atrophic appearance; (III) immunohistochemical results showed that the lesion contained three types of cells: pyloric gland, fundus gland, and foveolar epithelium; (IV) no recurrence was noticed during a follow-up of 9 months. In the future, the accumulation of cases may clarify clinicopathological characteristics.
Acknowledgments
We would like to thank Prof. Chengjun Zhou from the Department of Pathology, The Second Hospital, Shandong University, and Prof. Kuangi Fu from the Department of Endoscopy, Kanma Memorial Hospital, Japan for their sincere help in the histological diagnosis of the present case. We thank the Editage experts for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-197
Peer Review File: Available at https://dx.doi.org/10.21037/tcr-21-197
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-197). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cristallini EG, Ascani S, Bolis GB. Association between histologic type of polyp and carcinoma in the stomach. Gastrointest Endosc 1992;38:481-4. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Pezhouh MK, Park JY. Gastric pyloric gland adenoma. Arch Pathol Lab Med 2015;139:823-6. [Crossref] [PubMed]
- Pu Z, Ding X, Jiang H. Pathological diagnosis of gastric adenoma and early highly differentiated adenocarcinoma in Japan. Chinese Journal of Digestive Endoscopy 2020;37:11-4.
- Vieth M, Montgomery EA. Some observations on pyloric gland adenoma: an uncommon and long ignored entity! J Clin Pathol 2014;67:883-90. [Crossref] [PubMed]
- Vieth M, Kushima R, Borchard F, et al. Pyloric gland adenoma: a clinico-pathological analysis of 90 cases. Virchows Arch 2003;442:317-21. [Crossref] [PubMed]
- Kushima R, Vieth M, Borchard F, et al. Gastric-type well-differentiated adenocarcinoma and pyloric gland adenoma of the stomach. Gastric Cancer 2006;9:177-84. [Crossref] [PubMed]
- Zhang Y. The Expression and Significance of MUC2, MUC5AC, MUC6 and P53 in Gastric Cancer and Preinvasive Lesion. Capital Medical University, 2017.
- Park SY, Jeon SW, Jung MK, et al. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol 2008;20:966-70. [Crossref] [PubMed]
- Lim H, Jung HY, Park YS, et al. Discrepancy between endoscopic forceps biopsy and endoscopic resection in gastric epithelial neoplasia. Surg Endosc 2014;28:1256-62. [Crossref] [PubMed]
- Zhu LY, Dai J, Zhao YJ, et al. Endoscopic resection for gastric epithelial neoplasia: how to solve pathological discrepancy and achieve curative resection? J Dig Dis 2013;14:231-7. [Crossref] [PubMed]
- Takenawa H, Kurosaki M, Enomoto N, et al. Differential gene-expression profiles associated with gastric adenoma. Br J Cancer 2004;90:216-23. [Crossref] [PubMed]
- Zhang Y. To improve the diagnostic level of high-grade gastric intraepithelial neoplasia in gastric low-grade intraepithelial heoplasia. Chinese Journal of Clinical Gastroenterology 2017;29:51-4.
- Wu Y, Sang J, Zhou J. Analysis of risk factors for postoperative pathological upgraded of gastric low-grade intraepithelia neoplasia after endoscopic submucosal dissection. China Journal of Endoscopy 2020;26:1-6.
- Liu T. Application of endoscopic ultrasonography in the diagnosis and treatment of superdigestive submucosal tumors. Journal of Aerospace Medicine 2020;31:1335-6.
- Ling T, Chen G, Wang L. Clinical significance of endoscopic submucosal dissection in the treatment of high-grade intraepithelial neoplasia and early carcinoma of residual gastric glands. Chinese Journal of Digestive Endoscopy 2015;32:427-31.


