
Hemolymphangioma of the transverse mesocolon: a case report and literature review
Introduction
Hemolymphangioma is a congenital malformation of the vascular system, comprising both venous and lymphatic components. The interval from its prenatal origin to clinical presentation is quite variable but often the evolution of hemolymphangioma has been measured in decades. To progress from a diminutive cyst to a huge cystic tumor, it is almost never aggressive (1). Review of the English literature through January 2021 revealed only a limited number of hemolymphangioma cases involving the digestive tract organs such as the pancreas, liver, stomach, duodenum, small and large intestine. The intent of this case report and literature review was to highlight the key aspects of presentation, organ involvement, imaging, histopathological characteristics, and treatment of hemolymphangioma involving the gastrointestinal tract. We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-176).
Case presentation
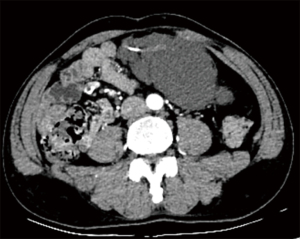
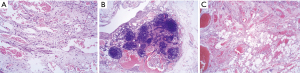
A 61-year-old male patient was admitted to the Second Hospital of Lanzhou University due to a 2-week history of intermittent left lower back pain. Subsequently, the pain was noted to include the left lower abdomen, and initially, no obvious cause was identified. The patient had noted a history of abdominal distension, but denied diarrhea, nausea, vomiting, and fever. There was no history of abdominal trauma, prior surgery or significant recent weight change. The patient had enjoyed normal health, his father and children were alive and well. His mother died of stomach cancer, not thought to be hereditary. The patient was not taking any medications. Physical examination revealed only mild tenderness in the left lower abdomen, but with no rigidity or rebound tenderness. An approximately 10 cm soft mobile well-defined mass was palpable in the left lower abdomen. Routine blood tests, biochemical examinations, and tumor markers were unremarkable. Computed tomography (CT) with vascular enhancement confirmed a large cystic mass in the left abdominal cavity, which was highly suspected to be a hemolymphangioma. It could not be clearly differentiated from other space occupying lesions in the abdominal cavity, but required surgical excision and pathological examination to provide a definitive diagnosis (Figure 1). The differential diagnosis was limited to such lesions as mesenteric cyst, lymphangioma, enteric duplication cyst, omental cyst, or stromal cell tumor. The patient underwent laparoscopic exploration on October 14, 2020, during which the tumor was found to originate from the transverse mesocolon and was completely excised. Pathological diagnosis of mesenteric hemolymphangioma was confirmed (Figure 2). The patient's postoperative course was uneventful, and at the 3-month follow-up, the patient had returned to normal health with no sign of cyst recurrence. This study was approved by the Medical Ethics Committee of the Second Hospital of Lanzhou University. The ID/number of ethical approval is 2020A-279. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
We used the PubMed database to conduct a systematic review of existing medical literature, with “hemolymphangioma” as the key search word to retrieve all the studies published as of January 2021. After reviewing and summarizing each published article, we selected 25 original studies with 19 case reports describing hemolymphangioma originating from the liver, stomach, duodenum, small intestine, colon, rectum, pancreas and other digestive tract organs (Table 1) (2-20). To summarize, hemolymphangioma principally occurred in adult patients with an average age of 42 years (range, 3–70 years), with 84% (16/19) over the age of 20. It is more common in female patients with a male to female ratio of 1:1.38 (8/11). The anatomical locations were: pancreas 37% (7/19), small intestine 31% (6/19), rectum 11% (2/19), and stomach, duodenum, liver, and greater omentum each with 5% (1 case each). These patients typically presented with either abdominal pain or blood in the stool, but these symptoms could persist for weeks or months, complicated by repeated episodes of blood loss and significant iron-deficiency anemia. Additionally, over time, symptoms of bloating, compression, and even obstruction became evident as the mass slowly but progressively enlarged. The most common physical signs were abdominal tenderness and a palpable abdominal mass.
Table 1
| Case/number | Publication year | Age (years)/sex | Localization | Preoperative diagnosis | Size (cm) | Chief complaint | Treatment | Physical Examination | imaging findings | Follow-up (months) | Recurrence | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2003 | 53/F | Pancreas | Space-occupying lesion of the head of pancreas | 4×3 | Abdominal pain and weight loss of 3 kg | Pancreatoduodenectomy with jejunostomy | Epigastric and right hypochondrium pain | Ultrasonography (US) showed a polycystic mass by the right renal pelvis. Computed tomography (CT) and magnetic resonance imaging (MRI) demonstrated a heterogeneous mass next to the head of the pancreas that partially compressed the right renal pelvis | NA | Not reported | Favourable |
| 3 | 2008 | 53/M | Pancreas | The pancreatic neoplasm | NA | Severe anemia due to gastrointestinal bleeding | Pylorus preserving pancreatoduodenectomy was performed | Anemic appearance with distension and pain in the upper abdomen | CT revealed a heterogenous mass at the pancreatic head and suspected invasion to the duodenal wall. Ultrasonography showed a huge mass at the pancreatic head with a mixture of high and low echoic areas | 12 | Not reported | Favourable |
| 4 | 2019 | 20/F | Pancreas | Large retroperitoneal tumor | 18×16×12.5 | A mass in abdominal cavity and epigastric discomfort about a week | Along the surface of the duodenum and pancreas, tumor (including partial transverse mesocolon and greater omentum) excision was performed. | A great abdominal mass | Abdominal computed tomography demonstrates a large tumor behind the peritoneum, possibly from the pancreas, compressing the duodenum with a polycystic structure and partial blood flow | 26 | Not reported | Favourable |
| 5 | 2011 | 68/M | Stomach | Submucosal benign cystic lesion | 4.5×3.1 | Mild epigastric discomfort 3 months after meals | Under EUS guidance, successfully excised | Not complain of any other symptoms | The undisturbed CT scan of the upper abdomen showed a well-defined, uniform, low-attenuation mass near the lesser curvature of the posterior wall of the stomach. Contrast-enhanced CT scan showed no obvious enhancement in the arterial phase and portal vein phase, but slight enhancement in the 2-minute delay scan | 18 | Not reported | Favourable |
| 6 | 2012 | 57/F | Small intestine | Small intestinal tumor | 5.0×4.0 | Recurrent melena more than 2 months | Partial intestinal resection | Not reported | Enteroscopy showed a gray mass with ulcers and erosion in the small intestine 30 cm distal to the flexor tendon | 12 | Not reported | Favourable |
| 7 | 2013 | 37/M | The rectum | Rectal cancer | 20×8×8 | Rectal bleeding and tenesmus | Low anterior resection of the rectosigmoid colon with handsewn transanal colo-anal anastomosis | Mild tenderness on the left lower quadrant | Colonoscopy revealed an extensive hypervascular submucosal lesion arising from the rectosigmoid junction colon to the distal edge of the anus. Endoscopic ultrasonography demonstrated an extensive anechoic mass with clear edge. Magnetic resonance imaging (MRI) showed a significant thickness of the rectal wall, extending to the distal edge of the anus, with a narrowing lumen | 12 | Not reported | Favourable |
| 8 | 2013 | 39/F | Pancreas | Mucinous cystadenoma or cystadenocarcinoma | 10×7 | Abdominal pain one day | Pancreatic body and tail combined with spleen resection | Slight tenderness in the left lower abdomen without rebound pain | The boundary is clear, cystic and solid, with a cable-like septum in the center of the cystic area. There is no change in enhanced CT images | NA | Not reported | Favourable |
| 9 | 2014 | 24/F | Duodenum | Duodenal mass | 4.0×1.5 | Severe and undetermined anemia | A local wide excision of the tumor | Not complain of any other symptoms | Magnetic resonance demonstrated a solid, polypoid mass (40 mm ×15 mm) at the lateral wall of the second/third portion of the duodenum with mild contrast enhancement, with no evidence of ampullary obstruction or periduodenal tissue infiltration | 4 | Not reported | Favourable |
| 10 | 2014 | 57/F | Pancreas | The pancreatic neoplasm | 7.8×6.0 | Epigastric discomfort for 10 days | A wide local resection of the tumor | Mild pain in the left hypochondrium without rebound tenderness | Abdominal computed tomography (CT) showed a cystic–solid tumor with an irregular shape, in the neck and body of the pancreas. The tumoral cystic wall and its internal division could be seen intensified on contrast-enhanced CT images compared with those on precontrast images | 2 | Not reported | Favourable |
| 11 | 2016 | 3/M | Greater omentum | Intraperitoneal benign cystic lesion | 20×15×6 | Mild but progressively increasing abdominal pain around umbilical region for 2 days | Abdominal laparotomy followed by surgical excision | Abdominal swelling, no tenderness or rebound tenderness | Abdominal computed tomography (CT) scan revealed a large intraperitoneal mass occupying almost all of the abdominal cavity and pelvis | 6 | Not reported | Favourable |
| 12 | 2017 | 57/M | Rectum | Rectal hemangioma | 25 cm long lesion | Massive rectal bleeding (rectorrhagia) for 5 months | Whole of the rectum and part of the sigmoid colon were excised and sigmoid-anus anastomosis was done. | On rectal examination, fresh blood was seen around anal region and soft mass was felt on digital rectal examination | Contrast-enhanced CT showed homogeneous thickening of the intestinal wall, uneven enhancement in the venous phase, and lesions extending from the distal sigmoid colon to the entire rectum | 6 | Not reported | Favourable |
| 13 | 2017 | 42/F | Liver | Solid focal liver lesion | 11.6×16.5 | Right upper abdominal weakness and acute abdominal pain for 2 months | Right hemihepatectomy was conducted | a large abdominal mass and apparent conjunctival pallor | Abdominal computed tomography revealed an enormous multilocular cystic mass located at the right lobe of the liver, measuring 11.6×16.5 cm | NA | Not reported | Favourable |
| 14 | 2017 | 45/F | Jejunum | Upper gastrointestinal hemorrhage | 8-cm long | Recurrent melena for about a year | A 15-cm segment of jejunum was resected with primary anastomosis | Not reported | Video capsule endoscopy showed a zone of lymphangiectasias with red blood in the proximal jejunum | NA | Not reported | Not reported |
| 15 | 2018 | 30/F | Pancreas | Abdominal neoplasm | 12×10×7.5 | Abdominal distension and an epigastric mass about 3 weeks | Body and tail pancreatectomy combined with middle colic artery and vein resection were performed | A soft mass in the upper abdomen | Computed tomography revealed a large multilocular cystic tumor in the neck and body of the pancreas | 24 | Not reported | Favourable |
| 16 | 2018 | 28/M | Pancreas | A retroperitoneal mature liposarcoma or ganglioneuroma | 8.0×10.0 | Right upper abdominal pain for 2 days | Pylorus preserving pancreatoduodenectomy was performed | A soft abdomen, no abdominal varicose veins, and a mass approximately 8.0×10.0 cm2 at the right upper quadrant that had an unclear border, poor activity, and tenderness (but no rebound tenderness) | Plain CT showed a cystic-solid mass of mixed density with a size of approximately 12 cm in front of the right kidney and behind the pancreatic head. Enhanced CT showed that the solidified part of the lesion was slightly strengthened, and the lesion’s boundary with the surrounding adipose tissues was unclear as it partially wrapped around the duodenum. MRI showed slightly high intensity and scattered low intensity on T1-weighted imaging (T1WI) and high/low mixed intensity on T2-weighted imaging (T2WI) | NA | Not reported | Favourable |
| 17 | 2018 | 70/M | Small intestine | The small intestine neoplasm | 2.0×1.7×1.2 | A 3-year history of iron deficiency anemia with occasional dark stool | Laparoscopic small bowel resection | Not complain of any other symptoms | Antegrade double-balloon enteroscopy was carried out, which demonstrated a 20 mm raised, granular lesion with white and thickened villi located 120 cm distal to the ligament of Treitz | NA | Not reported | Not reported |
| 18 | 2019 | 20/F | Jejunum | Stomach neoplasm | 6×4×3 | Intermittent anemia 7 years, black stool 1 month | Laparotomy | Nemic appearance and the laboratory tests showed iron deficiency anemia (hemoglobin was 52 g/L) | Enhanced computer tomography scan showed a low-density mass sized approximately 6×3×4 cm in the middle left part of the abdomen, with partial bowel dilatation | NA | Not reported | Not reported |
| 19 | 2019 | 55/F | Jejunum | Jejunal space-occupying lesion | 3.×3;2×2 | Discomfort in the right upper abdomen for 2 months | Laparotomy | mild tenderness on the right upper abdominal quadrant, with no rebound tenderness, and no abdominal mass | CT demonstrated a space-occupying lesion in proximal jejunum with calcium deposition, which had exhibited enhancement after contrast injection | 6 | Not reported | Favourable |
| 20 | 2020 | 42/M | Small intestinal | Gastric ulcer and anemia | NA | Repeated episodes of melena, dizziness, fatigue, decreased athletic ability for more than 2 months | Enteroscopic injection sclerotherapy | Anemic face and upper abdominal tenderness | Capsule endoscopy revealed a prominent lesion in the jejunum about 150 cm from the distal end of the Treitz ligament | 12 | Not reported | Favourable |
NA, the data were not available.
Hemolymphangioma, is a rare, congenital, benign malformation of the vascular system, histologically characterized by cystic dilated lymphatic and blood vessels. It originates from mesenchymal tissues, and bleeds easily with fewer clear lymphatic vessels (13). Hemolymphangiomas can be classified as primary and secondary according to the cause of the disease, with the former being considered a congenital obstruction of the venolymphatic communication between dysembryoplastic vascular tissue and the systemic circulation (11). The latter is caused by poor lymphatic drainage resulting from surgery or trauma (21,22), which is extremely rare in clinical practice. The clinical manifestations of hemolymphangioma vary depending on the size and location of the tumor. The variability of symptoms results from pressure exerted by the large cystic mass on adjacent structures, or the occurrence of complications, such as bleeding, perforation, torsion, or rupture of the tumor itself (17). In actual clinical practice, a preoperative diagnosis specifically of hemolymphangioma is virtually impossible due to its rarity, lack of characteristic imaging features or typical clinical manifestations.
Hemolymphangiomas occur predominantly in the pancreas, spleen and lower limbs, but rarely in the gastrointestinal tract. A clinical diagnosis of an intraperitoneal cystic mass is most often established on the basis of imaging. Both CT and magnetic resonance imaging (MRI) are quite valuable for determining the extent and possible invasion of tumors in general thereby facilitating preoperative surgical strategies (23). Each has its own particular advantages, and they are often complementary. A CT with vascular enhancement can identify areas of a tumor that are highly vascular or abut against important vessels. Hemolymphangiomas are a combination of dilated veins, dilated lymphatic vessels, and normal interstitial tissue. The signal for the cystic fluid by MRI examination is also related to the ratio of blood vessels to lymphatic vessels in the tumor (24). Accordingly, the T1W1 signal is mainly low or slightly low, whereas the T2W1 signal is mainly high.
As noted, abdominal hemolymphangiomas are principally manifested as space-occupying lesions in the abdomen, mainly presenting with compression symptoms, and should be differentiated from other such lesions, such as gastrointestinal stromal tumors, simple lymphangioma, and peritoneal pseudomucus. However, the final diagnosis requires pathological examination. Gastrointestinal stromal tumors are the most common stromal tumors of the gastrointestinal tract. They can also occur in the omentum, mesenteric, and retroperitoneum. They tend to grow exogenously. The histological types are mainly spindle cells or epithelioid cells (25,26). Many gastrointestinal stromal tumors show considerable cystic changes and sometimes intratumoral bleeding (27). Immunohistochemical staining is characterized by a diffuse and strong positive reaction for cd117, a positive reaction for CD34, and a negative reaction for S100 protein and desmin (28). Lymphangioma is a benign multilocular cystic mass that can occur anywhere in the abdominal cavity and is hidden between structures. Ultrasound can help distinguish intestinal duplication cysts from other mesenteric and omental cysts in the abdomen, the key sign being the double intestinal wall on the mesenteric side. Pathologically, a lymphangioma is a large, multi-cavity, thin-walled tumor, whose content is mainly chyle, but it may also be serous or hemorrhagic. Histologically, there are dilated endothelial cells arranged in the dilated lymphatic lumen (29).
Hemolymphangiomas are usually soft, have clear boundaries, exhibit polycystic changes in the cyst, and contain blood and lymph vessels in the cyst fluid. The hemolymphangioma specimen from the current case showed multi-nodular protrusions on the surface. The cut surface was grayish-yellow and covered with multiple small cysts. The maximum diameter of the cyst was approximately 12.5 cm, which was consistent with the appearance of a hemolymphangioma. The diagnosis of hemolymphangioma is established on postoperative pathology. A large number of expanded blood vessels and lymphatic vessels are characteristically observed microscopically. Moreover, hemolymphangiomas can be further distinguished through immunohistochemistry. Both vascular and lymphatic endothelial cells express CD31 and CD34, whereas D2-40 is only expressed in lymphangioma and some malignant vascular tumors. Such indicators can help solidify the diagnosis. Tumor cells in the current case expressed CD34 (vascular and interstitial +) and D2-40 (lymphatic vessels +), thereby adding strong evidence for a pathological diagnosis of vascular lymphangioma. Complete resection provides optimal treatment, with low recurrence rates (30). Hemolymphangioma recurrence is strongly associated with the specific location of the tumor and the completeness of surgical resection. Published studies have reported that 10–27% of completely resected tumors exhibit recurrence, whereas 50–100% of only partially resected tumors recur. Non-surgical treatments, including cryotherapy, laser therapy, radiation therapy, and local injection of a sclerosing agent, have also been utilized (31). In conclusion, the present case involving an elderly male patient with hemolymphangioma provides a concise, valuable vignette to summarize this lesion. He presented with abdominal pain and a palpably soft, mobile mass, further characterized by CT scan as a cystic, noninvasive intraperitoneal benign-appearing tumor. It was completely resected, and with the combination of standard histopathology and immunohistochemistry, the diagnosis of hemolymphangioma was established. The patient recovered and had an excellent prognosis. With symptoms such as abdominal space occupation, abdominal pain and obstruction, and when common benign and malignant tumors in the abdominal cavity have been excluded, the possibility of a gastrointestinal venolymphatic cystic tumor should be considered.
Acknowledgements
Thanks to EditSprings (https://www.editsprings.com/) experts for their language services.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE checklist. Available at https://dx.doi.org/10.21037/tcr-21-176
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-176). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of the Second Hospital of Lanzhou University (No:2020A-279). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kosmidis I, Vlachou M, Koutroufinis A, et al. Hemolymphangioma of the lower extremities in children: two case reports. J Orthop Surg Res 2010;5:56. [Crossref] [PubMed]
- Balderramo DC, Di Tada C, de Ditter AB, et al. Hemolymphangioma of the pancreas: case report and review of the literature. Pancreas 2003;27:197-9. [Crossref] [PubMed]
- Toyoki Y, Hakamada K, Narumi S, et al. A case of invasive hemolymphangioma of the pancreas. World J Gastroenterol 2008;14:2932-4. [Crossref] [PubMed]
- Sun LF, Ye HL, Zhou QY, et al. A giant hemolymphangioma of the pancreas in a 20-year-old girl: a report of one case and review of the literature. World J Surg Oncol 2009;7:31. [Crossref] [PubMed]
- Handra-Luca A, Montgomery E. Vascular malformations and hemangiolymphangiomas of the gastrointestinal tract: morphological features and clinical impact. Int J Clin Exp Pathol 2011;4:430-43. [PubMed]
- Fang YF, Qiu LF, Du Y, et al. Small intestinal hemolymphangioma with bleeding: a case report. World J Gastroenterol 2012;18:2145-6. [Crossref] [PubMed]
- Chen G, Cui W, Ji XQ, et al. Diffuse hemolymphangioma of the rectum: a report of a rare case. World J Gastroenterol 2013;19:1494-7. [Crossref] [PubMed]
- Dong F, Zheng Y, Wu JJ, et al. Hemolymphangioma: a rare differential diagnosis of cystic-solid or cystic tumors of the pancreas. World J Gastroenterol 2013;19:3520-3. [Crossref] [PubMed]
- Antonino A, Gragnano E, Sangiuliano N, et al. A very rare case of duodenal hemolymphangioma presenting with iron deficiency anemia. Int J Surg Case Rep 2014;5:118-21. [Crossref] [PubMed]
- Figueroa RM, Lopez GJ, Servin TE, et al. Pancreatic hemolymphangioma. JOP 2014;15:399-402. [PubMed]
- Pandey S, Fan M, Chang D, et al. Hemolymphangioma of Greater Omentum: A Rare Case Report. Medicine (Baltimore) 2016;95:e3508. [Crossref] [PubMed]
- Pandey S, Fan M, Zhu J, et al. Unusual cause of 55 years of rectal bleeding: hemolymphangioma (a case report). Medicine (Baltimore) 2017;96:e6264. [Crossref] [PubMed]
- Hu HJ, Jing QY, Li FY. Hepatic Hemolymphangioma Manifesting as Severe Anemia. J Gastrointest Surg 2018;22:548-9. [Crossref] [PubMed]
- Blanco Velasco G, Tun Abraham A, Hernández Mondragón O, et al. Hemolymphangioma as a cause of overt obscure gastrointestinal bleeding: a case report. Rev Esp Enferm Dig 2017;109:213-4. [PubMed]
- Zhang Z, Ke Q, Xia W, et al. An Invasive Hemolymphangioma of the Pancreas in a Young Woman. Comb Chem High Throughput Screen 2018;21:798-800. [Crossref] [PubMed]
- Chen Q, Xia J. A giant hemolymphangioma of the pancreas: A case report and literature review. Medicine (Baltimore) 2018;97:e12599. [Crossref] [PubMed]
- Iwaya Y, Streutker CJ, Coneys JG, et al. Hemangiolymphangioma of the small bowel: A rare cause of chronic anemia. Dig Liver Dis 2018;50:1248. [Crossref] [PubMed]
- Yang J, Zhang Y, Kou G, et al. Jejunum Hemolymphangioma Causing Refractory Anemia in a Young Woman. Am J Gastroenterol 2020;115:810. [Crossref] [PubMed]
- Teng Y, Wang J, Xi Q. Jejunal hemolymphangioma: A case report. Medicine (Baltimore) 2020;99:e18863. [Crossref] [PubMed]
- Xiao NJ, Ning SB, Li T, et al. Small intestinal hemolymphangioma treated with enteroscopic injection sclerotherapy: A case report and review of literature. World J Gastroenterol 2020;26:1540-5. [Crossref] [PubMed]
- Zhang X, Sheng X, Liu F, et al. Hemolymphangioma of the chest wall: A rare case report. Oncol Lett 2012;3:816-8. [PubMed]
- Li Y, Pang X, Yang H, et al. Hemolymphangioma of the waist: A case report and review of the literature. Oncol Lett 2015;9:2629-32. [Crossref] [PubMed]
- Pan L, Jian-Bo G, Javier PTG. CT findings and clinical features of pancreatic hemolymphangioma: a case report and review of the literature. Medicine (Baltimore) 2015;94:e437. [Crossref] [PubMed]
- Mao CP, Jin YF, Yang QX, et al. Radiographic findings of hemolymphangioma in four patients: A case report. Oncol Lett 2018;15:69-74. [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438:1-12. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- King DM. The radiology of gastrointestinal stromal tumours (GIST). Cancer Imaging 2005;5:150-6. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Levy AD, Cantisani V, Miettinen M. Abdominal lymphangiomas: imaging features with pathologic correlation. AJR Am J Roentgenol 2004;182:1485-91. [Crossref] [PubMed]
- Woo YS, Joo KR, Kim KY, et al. Unusual presentation of cystic lymphangioma of the gallbladder. Korean J Intern Med 2007;22:197-200. [Crossref] [PubMed]
- Kosmidis I, Vlachou M, Koutroufinis A, et al. Hemolymphangioma of the lower extremities in children: two case reports. J Orthop Surg Res 2010;5:56. [Crossref] [PubMed]


