
Bioinformatics analysis for the biomarkers of the tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC) are recognized as an aggressive malignant tumor with poor overall survival (OS) worldwide (1). Accordingly, most HNSCC patients are often diagnosed at an advanced stage (2). Despite advances in multimodality treatments (MDTs), the prognosis for HNSCC patients still needs to be improved (3). Immunotherapy has revolutionized the treatment of cancer, and is regarded as a potential alternative for patients with advanced HNSCC, especially in the use of programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) immune checkpoint inhibitors (4). However, immunotherapy strategies are successful in only a subset of advanced HNSCC patients (4).
Growing evidence suggests that the tumor microenvironment (TME), especially the accumulation of tumor-infiltrating lymphocytes (TILs), is important for the prognosis of various types of cancer (5-7). TILs often fail to eliminate cancer cells resulting from an immunosuppressive TME characterized by the expression of multiple inhibitory receptors (4,5). Effective cancer immunotherapy greatly depends on the cellular burden, heterogeneity, status and phenotypes of immune cells in the TME (8,9). Therefore, a comprehensive assessment of TILs can provide important clinicopathological and prognostic information, which might be of value in predicting and improving the response to immunotherapy. Accordingly, HNSCC has been observed to have a high infiltration of immune cells, especially the infiltration with high levels of TILs (10,11). A simple evaluation for the density and distribution of TILs in HNSCC is obviously insufficient. Besides, controversial results have been reported because of the limited sample sizes. Consequently, the biological roles, clinical significance for TILs in HNSCC have not been completely established, especially concerning the heterogeneity and correlations of various TILs. What’s more, the PD-1/PD-L1 axis has been reported to be tightly correlated to the immunological profile of TILs in a variety of cancers (6,11). In this study, a series of bioinformatics analysis were performed for the expression pattern of cell surface markers of different TILs, CD4 (a marker for T-Helper cells), CD8 (a marker for cytotoxic T cells), CD20 (a marker for B cells), and CD56 (a marker for NK cells), in HNSCC. The study aimed to present a preliminary study to demonstrate the correlations and heterogeneity of different TILs subpopulations and PD-1/PD-L1 axis with a relatively large sample size based on the TCGA primary HNSCC cohort. We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-408).
Methods
Gene expression analysis
Expression profiles of the involved molecules were analyzed based on the TCGA HNSC cohort (604 cases) using the UCSC Xena Browser (http://xena.ucsc.edu) (12). Herein, only primary HNSCC cases (528 cases) were filtered and included for further analysis. Heat-maps (red-black-green) of defined gene sets were generated online, and detailed data were downloaded to investigate the expression relationships between involved genes.
Clinicopathological analysis
The clinicopathological characteristics of the involved genes were investigated based on the primary HNSCC cases of the TCGA HNSC cohort (13). We downloaded and analyzed the detailed data for the expression level, pathological stage, pathological tumor size, pathological nodal status, histological grade, perineural invasion, lymphovascular invasion presence, nodal extracapsular spread, EGFR amplification status, and human papilloma virus (HPV) status. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Prognostic analysis
The prognostic significance based on the expression level of the involved genes was evaluated for HNSCC patients by using an online tool, Kaplan-Meier Plotter (http://www.kmplot.com) (14). OS and recurrence-free survival (RFS) were analyzed for all patients based on the expression level of each gene with a hazard ratio (HR) with 95% confidence intervals (CIs) and log-rank P values.
Functional prediction analysis
Bioinformatics predictions for coexpression, physical interactions, pathways, and genetic interactions for involved genes was performed by using GeneMANIA (http://www.genemania.org) (15). Cytoscape (version 3.6.1, USA) was used to present the networks (15).
Statistical analysis
Statistical analyses were conducted with SPSS 20.0 software (NY, USA). The coexpression relationships between the involved genes were evaluated by RxC crosstable analyses and chi-squared tests (13). Chi-squared tests were used to assess the statistical significance of the correlation between the gene expression and the clinicopathological variable for each involved gene (13). Statistically, P<0.05 (two-sided) were considered significant.
Results
Expression pattern of different TIL-related molecules based on the TCGA primary HNSCC cohort
The major TIL subpopulations (T cells, B cells, and NK cells) were investigated in this study. Herein, the expression levels of CD4 (a marker for T-helper cells), CD8 (a marker for cytotoxic T cells), CD20 (a marker for B cells), and CD56 (a marker for NK cells) were investigated in HNSCC (Figure 1A). In addition, the expression levels of PD-1 and PD-L1 were also analyzed (Figure 1B). Significant correlations were observed among the expression levels of CD4, CD8, and CD20, and the expression level of CD56 was only observed to be significantly correlated with the expression level of CD4 based on the TCGA primary HNSCC cohort (Figure 1C). Accordingly, PD-L1 and PL1 were significantly coexpressed in HNSCC (Figure 1D). We also observed that the expression of PD-L1 or PD-1 was significantly correlated with the expression of CD4, CD8, and CD20, but not CD56 based on the TCGA primary HNSCC cohort (Figure 1E,F).
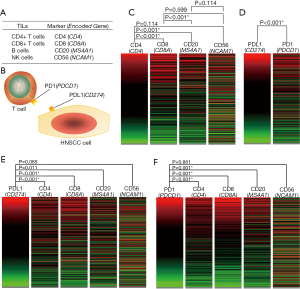
Clinical values for TIL-related molecules based on the TCGA primary HNSCC cohort
As summarized in Table 1, the clinicopathological significance was analyzed based on the expression levels of CD4, CD8, CD20, CD56, PD-1, and PD-L1 according to the TCGA primary HNSCC cohort. For the evaluation of pathological tumor size, significantly negative correlations for the molecules CD20, CD56, and PD-1 were identified. PD-1 expression was observed to be positively correlated to the pathological nodal status. For evaluation of histological grade, significantly positive correlations for the molecules CD4, CD8, and PD-1 were identified. Regarding perineural invasion, we observed significantly positive correlations for the molecules CD20 and PD-1, and a significantly negative correlation for the molecule CD56. CD20 expression was observed to be negatively correlated with nodal extracapsular spread. Accordingly, no significant correlation was observed between EGFR amplification status and the expression levels of CD4, CD8, CD20, CD56, PD-1, or PD-L1. In addition, for the evaluation of HPV status, we observed significantly positive correlations for the molecules CD8, CD20 and PD-1, and a significantly negative correlation for the molecule CD56.
Table 1
| Clinicopathological features | P value | |||||
|---|---|---|---|---|---|---|
| CD4 | CD8 | CD20 | CD56 | PD-1 | PD-L1 | |
| Pathological stage (I+II/III+IV) | 0.116 | 0.675 | 0.602 | 0.088 | 0.176 | 0.052 |
| Pathological T (T1+T2/T3+T4) | 0.086 | 0.117 | 0.022† | 0.008† | 0.007† | 0.286 |
| Pathological N (N0/N1+) | 0.239 | 0.243 | 0.575 | 0.688 | 0.04‡ | 0.974 |
| Histological Grade (G1+G2/G3+G4) | 0.019‡ | 0.011‡ | 0.967 | 0.499 | 0.04‡ | 0.797 |
| Perineural invasion (yes/no) | 0.598 | 0.946 | 0.001† | 0.006‡ | 0.037† | 0.368 |
| Lymphovascular invasion present (yes/no) | 0.584 | 0.455 | 0.707 | 0.464 | 0.954 | 0.267 |
| Nodal extracapsular spread (yes/no) | 0.457 | 0.248 | 0.03† | 0.799 | 0.639 | 0.720 |
| EGFR amplification status (amplified/unamplified) | 0.096 | 0.238 | 0.679 | 0.701 | 0.182 | 0.701 |
| HPV status by FISH testing (positive/negative) | 0.145 | <0.001‡ | <0.001‡ | 0.002† | <0.001‡ | 0.600 |
| HPV status by p16 testing (positive/negative) | 0.098 | 0.001‡ | 0.003‡ | <0.001† | 0.002‡ | 0.962 |
†, negative correlation; ‡, positive correlation. TILs, tumor-infiltrating lymphocytes; HNSCC, head and neck squamous cell carcinoma; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; HPV, human papilloma virus.
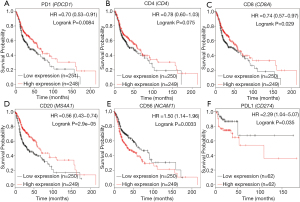
The prognostic values for the expression of TIL-related genes, PDCD1 and CD274 were investigated for patients with HNSCC using Kaplan-Meier Plotter. Accordingly, increased expression of PD-1 was significantly correlated with improved OS for HNSCC patients (Figure 2A). No significant correlation was found between increased expression of CD4 and improved OS (Figure 2B). Additionally, increased expression of CD8 and CD20 was shown to have a significant relationship with improved OS (Figure 2C,D). HNSCC patients with increased CD56 expression possessed obviously decreased OS (Figure 2E). Moreover, increased expression of PD-L1 was significantly correlated with decreased RFS in HNSCC patients (Figure 2F).
IL2RB might work as a hub gene among the TILs in HNSCC
GeneMANIA was used to construct co-expression (Figure 3A), physical interactions, pathway, and genetic interaction networks of TIL-related markers, PDCD1, CD274, and their potentially related proteins. In the coexpression network (Figure 3A), the database identified IL2RB which was closely associated with TIL-related markers, PDCD1, and CD274.
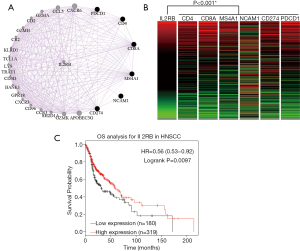
The gene alterations of IL2RB were investigated in HNSCC using cBioPortal, and four datasets were analyzed. We observed that the expression of IL2RB significantly correlated with the expression of CD4, CD8, CD20, CD56, PD-1, and PD-L1 based on the TCGA primary HNSCC cohort (Figure 3B). As summarized in Table 2, significantly negative correlations were observed between IL2RB expression and pathologic tumor size (P=0.006), and pathologic stage (P=0.013). In addition, a significantly positive correlation was observed between IL2RB expression and HPV status (P=0.009 for FISH testing, and P=0.025 for p16 testing). The prognostic value for the expression of IL2RB was investigated for patients with HNSCC using the Kaplan-Meier Plotter. Accordingly, increased expression of IL2RB was significantly correlated with improved OS for HNSCC patients (Figure 3C).
Table 2
| Variable | N | IL2RB expression (≤8.5) | IL2RB expression (>8.5) | P value |
|---|---|---|---|---|
| Pathologic T | 0.006† | |||
| T1+T2 | 184 | 80 | 104 | |
| T3+T4 | 273 | 155 | 118 | |
| Pathologic N | 0.692 | |||
| N0 | 176 | 95 | 81 | |
| N1+ | 244 | 126 | 118 | |
| Pathologic stage | 0.013† | |||
| I+II | 101 | 41 | 60 | |
| III+IV | 347 | 191 | 156 | |
| Neoplasm histologic grade | 0.068 | |||
| G1+G2 | 366 | 193 | 173 | |
| G3+G4 | 132 | 57 | 75 | |
| Lymphovascular invasion present | 0.574 | |||
| Yes | 123 | 69 | 54 | |
| No | 225 | 118 | 107 | |
| Pathological nodal extracapsular spread | 0.068 | |||
| Yes | 112 | 67 | 45 | |
| N0 | 244 | 120 | 124 | |
| Perineural invasion present | 0.246 | |||
| Yes | 169 | 96 | 73 | |
| N0 | 193 | 97 | 96 | |
| EGFR amplication | 0.061 | |||
| Amplified | 11 | 7 | 4 | |
| Unamplified | 16 | 4 | 12 | |
| HPV status by FISH | 0.009† | |||
| Positive | 21 | 3 | 18 | |
| Negative | 65 | 31 | 34 | |
| HPV status by p16 testing | 0.025† | |||
| Positive | 38 | 10 | 28 | |
| Negative | 73 | 36 | 37 |
†, negative correlation. HNSCC, head and neck squamous cell carcinoma; HPV, human papilloma virus.
Discussion
Cancer immunotherapy has greatly improved the treatment landscape for advanced cancer. Great efforts have also been made to introduce immunotherapy into the treatment of advanced HNSCC (4,10). Nevertheless, the therapeutic efficacy and clinical outcome of immunotherapy remain unsatisfactory and variable (4-6). In addition, the roles of TILs and their associations with PD-1/PD-L1 in HNSCC are not clear. Thus, there is a great need to investigate the biological roles and clinical significance of TILs in HNSCC, which might provide information to improve the outcome of immunotherapy in HNSCC. In this study, we observed significant correlations among CD4, CD8, and CD20 at the expression level, and the expression of PD-1/PD-L1 correlated well with CD4, CD8, and CD20, but not with CD56 in HNSCC. In the clinical evaluation, variable roles for the TILs were compared and demonstrated great heterogeneity in HNSCC. Having a clear knowledge of the different TILs is essential for conducting immunotherapy in HNSCC.
Tumor-infiltrating T cells are described as important immune components in the TME, and have been associated with the development and progression of cancers (16). In this study, the clinical significance of CD4 and CD8 was demonstrated in HNSCC. Generally, CD4+ Th cells can facilitate the antitumor activity of CTLs by enhancing clonal expansion at the tumor site, preventing activation-induced cell death and functioning as antigen-presenting cells (APCs) for CTLs (17,18). In HNSCC, we demonstrated a significant correlation between CD4 and CD8, CD20, CD56, PD-1, or PD-L1, indicating that CD4+ Th cells might exert complex roles in the TMEs of HNSCC. Clinicopathologically, the infiltration of CD4+ Th cells might worsen the histological grade of HNSCC. Prognostically, the infiltration of CD4+ Th cells might not directly affect the clinical outcomes of HNSCC patients. In contrast, CD8+ CTLs are the main anticancer effector cell subset in the TME, and play an essential role in mediating the response to chemotherapy (19,20). In addition, increased infiltration of CD8+ CTLs has been associated with improved clinical outcome in several types of cancer (19,21). In HNSCC, we found a significant correlation between CD8 and CD4, CD20, PD-1, or PD-L1. We observed that the infiltration of CD8+ CTLs might worsen the histological grade of HNSCC, but increased infiltration of CD8+ CTLs could improve the OS of HNSCC patients. For HNSCC cases with a high CD8+ CTL infiltration burden, it seems to be a potentially effective therapeutic approach to enhancing the tumor killing activity of CD8+ CTLs.
Tumor-infiltrating CD20+ B lymphocytes are important players in immune responses to cancer (22). The infiltration of CD20+ B cells has been linked mainly to good prognosis in different types of cancer (22). In the TME, B cells are the source of antibodies, which participate in tumor recognition and antibody-dependent cytotoxicity (22). In addition, B cells act as antigen-presenting cells and have the capacity to activate T cells to amplify the immune response (22,23). By secreting various cytokines, infiltrated B cells can also shape the immune response in an antitumor direction (22). In this study, a tight correlation between CD20+ B lymphocytes and CD4+ Th cells or CD8+ CTLs was indicated. In addition, significantly positive correlations between CD20 and PD-1/PD-L1 have also been indicated. In HNSCC, the increased infiltration of B cells obviously decreases the risk for pathological tumor size, perineural invasion, and nodal extracapsular spread. In addition, increased infiltration of CD20+ B lymphocytes could improve the OS of HNSCC patients.
Accordingly, NK cells make up a small portion of the TILs, and the importance of NK cells in the TME has been gradually recognized (24). The roles of NK-TILs are not limited to cell-mediated cytotoxicity in patients with cancer, and the distinctive function of NK-TILs has been demonstrated in several types of cancer (25). Consequently, the prognostic value of NK-TILs has been reported as variable in different types of cancer (24). To date, the clinical significance of NK-TIL infiltration in HNSCC has not been demonstrated. In this study, our data indicate that increased infiltration of NK-TILs obviously decreases the risk for pathological tumor size, but increases the risk for perineural invasion. Moreover, HNSCC patients with increased infiltration of NK-TILs possessed obviously decreased OS. In HNSCC, no significant correlations between CD56 and PD-1/PD-L1 were observed. These data indicate that great heterogeneity exists between NK-TILs and other types of TILs in HNSCC. There must be potential inhibitory signaling in HNSCC limiting the cytotoxicity of the infiltrated NK-TILs in HNSCC. Therefore, evaluating the infiltrating status of NK-TILs and decreasing the inhibitory signals imposed on the NK-TILs should not be neglected when performing immunotherapy for HNSCC patients.
In this study, the relationship between HPV status and TIL infiltration was also investigated. Our data indicate that a positive HPV status significantly correlates with increased infiltration of CD8+ CTLs and CD20+ B lymphocytes, decreased infiltration of NK-TILs, and increased expression of PD-1 in the TME of HNSCC. There is an increasing proportion of HNSCC patients resulting from high-risk HPV (22,26). Comparatively, HPV-driven HNSCC presents a more favorable clinical prognosis and better response to chemoradiation therapy (21,26). This difference might be explained by the increased infiltration of CD8+ CTLs and CD20+ B lymphocytes and decreased infiltration of NK-TILs.
By bioinformatics prediction, we demonstrated that IL2RB might work as a hub gene to regulate the infiltration of TILs and the expression of PD-1/PD-L1 in HNSCC. IL2/IL2Rβ signaling has been found to convey essential signals for key immune cells including T cells and NK cells (27). Therefore, IL2RB is considered a drug target to activate and empower immune therapy for HNSCC. Further investigation is still required to demonstrate and confirm the roles of IL2RB in the TME of HNSCC.
Our data suggest that PD-1/PD-L1 expression is associated with increased infiltration of CD4+ Th cells, CD8+ CTLs, and CD20+ B lymphocytes in HNSCC, indicating that the PD-1/PD-L1 pathway is a promising immunotherapeutic target for HNSCC. In 2016, the U.S. Food and Drug Administration approved the use of the PD-1 antibodies pembrolizumab and nivolumab in patients with recurrent or metastatic (R/M) HNSCC, especially for patience with chemotherapy resistance (4). To date, more drugs binding PD-1 or PD-L1 have been investigated and more clinical trials have been performed to acquire more rational strategies to benefit more HNSCC patients. In this study, we observed that PD-1/PD-L1 expression had no exact associations with the infiltration of NK-TILs. Accordingly, increased infiltration of NK-TILs increases the risk for perineural invasion, and increased infiltration of NK-TILs show a shorter OS. Therefore, enhancing the cytotoxicity of NK-TILs should also be involved to block inhibitory signaling and improve NK-based cancer immunotherapy in HNSCC. Till now, multiple phase I and phase II clinical trials testing NK-TIL checkpoint blockades against KIR and NKG2A as a therapy for hematological and solid tumors are ongoing (28). Targeting NK-TILs will be an important and necessary supplement to immunotherapy based on PD-1/PD-L1 in HNSCC.
Herein, we conducted a bioinformatics analysis to evaluate the TILrelated molecules based on the TCGA primary HNSCC cohort. However, one main limitation of this study that should not be ignored was the lack of external validation in independent clinical bio-samples. In the future, more experimental research and clinical studies should be conducted on the TILs in HNSCC, especially considering the correlations and heterogeneity of different TILs subpopulations and PD-1/PD-L1 axis. Conclusively, it seems to be necessary to develop a rational workflow to evaluate the TILs status for each HNSCC patients, which can be informative to predict prognosis and direct the immunotherapy for HNSCC patients.
Acknowledgments
Funding: This work was partially sponsored by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-408
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tcr-21-408
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-408). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer 2018;18:269-82. [Crossref] [PubMed]
- Ringash J. Survivorship and Quality of Life in Head and Neck Cancer. J Clin Oncol 2015;33:3322-7. [Crossref] [PubMed]
- Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol 2019;99:104460. [Crossref] [PubMed]
- Zhang J, Endres S, Kobold S. Enhancing tumor T cell infiltration to enable cancer immunotherapy. Immunotherapy 2019;11:201-13. [Crossref] [PubMed]
- D'Alterio C, Buoncervello M, Ieranò C, et al. Targeting CXCR4 potentiates anti-PD-1 efficacy modifying the tumor microenvironment and inhibiting neoplastic PD-1. J Exp Clin Cancer Res 2019;38:432. [Crossref] [PubMed]
- Miksch RC, Schoenberg MB, Weniger M, et al. Prognostic Impact of Tumor-Infiltrating Lymphocytes and Neutrophils on Survival of Patients with Upfront Resection of Pancreatic Cancer. Cancers 2019;11:39. [Crossref] [PubMed]
- Sanmamed MF, Chen L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2019;176:677. [Crossref] [PubMed]
- Hegde PS, Chen DS. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020;52:17-35. [Crossref] [PubMed]
- Cristina V, Herrera-Gómez RG, Szturz P, et al. Immunotherapies and Future Combination Strategies for Head and Neck Squamous Cell Carcinoma. Int J Mol Sci 2019;20:5399. [Crossref] [PubMed]
- Mandal R, Şenbabaoğlu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016;1:e89829. [Crossref] [PubMed]
- Goldman M, Craft B, Swatloski T, et al. The UCSC Cancer Genomics Browser: update 2015. Nucleic Acids Res 2015;43:D812-7. [Crossref] [PubMed]
- Xiao M, Liu L, Zhang S, et al. Cancer stem cell biomarkers for head and neck squamous cell carcinoma: A bioinformatic analysis. Oncol Rep 2018;40:3843-51. [Crossref] [PubMed]
- Györffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 2010;123:725-31. [Crossref] [PubMed]
- Montojo J, Zuberi K, Rodriguez H, et al. GeneMANIA: Fast gene network construction and function prediction for Cytoscape. F1000Res 2014;3:153. [Crossref] [PubMed]
- Thommen DS, Schumacher TN. T Cell Dysfunction in Cancer. Cancer Cell 2018;33:547-62. [Crossref] [PubMed]
- Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev 2008;222:129-44. [Crossref] [PubMed]
- Borst J, Ahrends T, Bąbała N, et al. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 2018;18:635-47. [Crossref] [PubMed]
- Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 2016;4:59. [Crossref] [PubMed]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486-99. [Crossref] [PubMed]
- Kim A, Han CJ, Driver I, et al. LILRB1 Blockade Enhances Bispecific T Cell Engager Antibody-Induced Tumor Cell Killing by Effector CD8+ T Cells. J Immunol 2019;203:1076-87. [Crossref] [PubMed]
- Lechner A, Schlößer HA, Thelen M, et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology 2019;8:1535293. [Crossref] [PubMed]
- Hollern DP, Xu N, Thennavan A, et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell 2019;179:1191-1206.e21. [Crossref] [PubMed]
- Triki H, Charfi S, Bouzidi L, et al. CD155 expression in human breast cancer: Clinical significance and relevance to natural killer cell infiltration. Life Sci 2019;231:116543. [Crossref] [PubMed]
- Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2016;16:7-19. [Crossref] [PubMed]
- Patel JJ, Levy DA, Nguyen SA, et al. Impact of PD-L1 expression and human papillomavirus status in anti-PD1/PDL1 immunotherapy for head and neck squamous cell carcinoma-Systematic review and meta-analysis. Head Neck 2020;42:774-86. [Crossref] [PubMed]
- Jounaidi Y, Cotten JF, Miller KW, et al. Tethering IL2 to Its Receptor IL2Rβ Enhances Antitumor Activity and Expansion of Natural Killer NK92 Cells. Cancer Res 2017;77:5938-51. [Crossref] [PubMed]
- Pomeroy EJ, Hunzeker JT, Kluesner MG, et al. A Genetically Engineered Primary Human Natural Killer Cell Platform for Cancer Immunotherapy. Mol Ther 2020;28:52-63. [Crossref] [PubMed]


