
Intersegmental plane simulation based on the bronchus-vein-artery triad in pulmonary segmentectomy
Introduction
In recent years, segmentectomy has been increasingly applied in the surgical treatment of early lung cancer, and its advantages have gradually emerged (1-5). Preoperative planning with three-dimensional (3D) reconstruction plays a key role in segmentectomy and has been accepted by many researchers (6-10). Thorough preoperative planning allows lesion resection with sufficient surgical margins. With the recent development of 3D reconstruction software, application of various software programs in the preoperative planning of segmentectomy has been reported; these methods achieve accurate delineation of the pulmonary artery, pulmonary vein, and bronchus to successfully guide surgery (6-10). However, great deficiencies persist in intersegmental plane (ISP) simulation. In most preoperative simulation procedures, the ISP is formed by connecting the intersegmental veins (judged by the operator based on clinical experience) using a 3D reconstructor. The disadvantage of this process is that it is greatly affected by the operator’s experience and thus has poor repeatability. We improved this process using the bronchus-vein-artery triad to simulate the ISP and judge the accuracy of ISP simulation. The process is not affected by the operator’s experience; thus, it has good repeatability and wide applicability. This study aimed to examine the value of the bronchus-vein-artery triad in segmentectomy for early peripheral lung cancer. The bronchus-vein-artery triad refers to use of the segmental bronchi to simulate the ISP preoperatively, the intersegmental vein to determine the accuracy of ISP simulation, and the segmental artery to present the ISP during surgery; these three segmental structures are fully used in the whole operation.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-822).
Methods
This is a retrospective controlled study. From January 2019 to July 2019, 120 patients with early-stage lung cancer underwent 3D imaging-guided single-port thoracoscopic segmentectomy (IQQA-3D; developed by IQQA-Chest, EDDA Technology, Princeton Junction, NJ, USA) at Department No. 1 of Thoracic Surgery at Fujian Medical University Fujian Union Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the institutional review board of Fujian Medical University Union Hospital (No. 2021KY112), which waived the need for informed consent of the patients.
The inclusion criteria were as follows: (I) patients with single or multiple peripheral pulmonary nodules who had undergone a single type of pulmonary segmental surgery; (II) nodules with a diameter of ≤2 cm; (III) a diagnosis of microinvasive or invasive adenocarcinoma; (IV) operative ranges that included single-port thoracoscopic segmentectomy, subsegmentectomy, or combined resection of different segment parts (segment, subsegment, or sub-subsegment), with mediastinal lymph node dissection or sampling; and (V) surgery performed under guidance of the IQQA-3D system, with or without ISP simulation and nodule analysis.
ISP simulation involved marking the bronchi of the target lung segment with different colors using 3D reconstruction software; the ISP was automatically simulated according to the size, shape, course, and other information of the bronchi.
Nodule analysis refers to adequate margins. According to the requirements of a safe tumor resection margin described in National Comprehensive Cancer Network (NCCN) guidelines (11), the nodule was used as the center of a sphere with a spherical margin equal to 2 cm or equal to lesion diameter. The spherical margin was shown in the 3D image.
The exclusion criteria were as follows: (I) presence of severe emphysema; (II) severe pleural adhesions discovered during surgery; (III) history of other operations, radiotherapy, or chemotherapy within the previous 3 months; and (IV) incomplete preoperative planning or insufficient data for analysis.
Case grouping
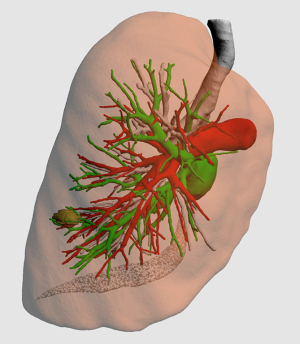
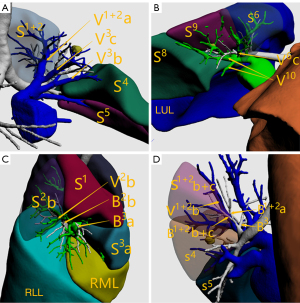
Patients were divided into two groups according to ISP and nodule analysis: the ISP group and the non-ISP group. In the non-ISP group, only 3D reconstruction of the three structures of the target lobes and localization of pulmonary nodules were conducted (Figure 1). ISP simulation and pulmonary nodule analysis were not performed. The surgical range and procedures for segmentectomy were formulated on the basis of this information.
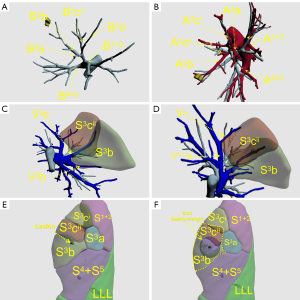
In the ISP group, 3D reconstruction of the target bronchus, pulmonary artery, and pulmonary vein (Figure 2A-2D); ISP simulation; pulmonary nodule localization; and nodular analysis were performed using the IQQA-3D system. The generated spherical boundary and the simulated ISP were used to determine the range of surgical resection that included the spherical boundary (Figure 2E,2F). Next, intersegmental veins that needed to be preserved, as well as target segmental bronchi, arteries, and internal veins that needed to be dissected, were analyzed. The procedure was formulated in accordance with the anatomical structures.
Data collection
Collected data included patient age, sex, 3D preoperative planning results (number of intersegmental veins used to evaluate the ISP and number of intersegmental veins located on the ISP), consistency between the simulated and actual ISPs, number of resected nodules, maximum lesion diameter, pulmonary function, postoperative pathology, operation characteristics (resection range, mediastinal lymph node dissection, operation time, and intraoperative blood loss), and postoperative recovery (duration of chest drainage, length of postoperative hospital stay, and postoperative complications).
Evaluation of the accuracy of ISP simulation
Using the 3D reconstruction system, the bronchus, pulmonary artery, and pulmonary vein of the pulmonary segment were reconstructed according to information from computed tomography (CT) images. The ISP was simulated according to the characteristics of the bronchus, such as its course, size, and shape. This process only involved bronchial information. Anatomically, the intersegmental veins ran along the ISP; therefore, ISP simulation was considered accurate if the simulated intersegmental vein fell on the simulated ISP.
Surgical procedures
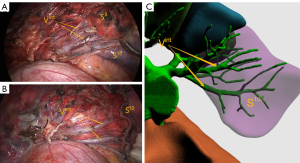
All patients underwent single-port thoracoscopic surgery. Patients were placed in the lateral decubitus position. An incision was made at the fifth intercostal space on the midaxillary line for basal segmentectomy, and at the fourth intercostal space for other segmentectomy. The length of the incision was approximately 4 cm. Dissection of target segmental arteries, bronchi, and veins was carried out in accordance with preoperative planning. Segmental arteries and veins were generally ligated with #1 or #4 suture and were cut with an ultrasonic knife; segmental bronchi were transected with a stapler or ligated by #1 or #4 suture. Next, the intersegmental boundary was revealed with the pure oxygen inflation-deflation method (6), and the ISP was divided with a combination of an ultrasonic knife and a linear cutting stapler. In the process, intersegmental veins were seen running along the ISP (Figure 3), and agreement between the simulated ISP and the actual ISP was evaluated in the ISP group (Figure 4). After resection, the margins were examined. Wedge resection of adjacent lung tissue was performed if the gross margin was insufficient.
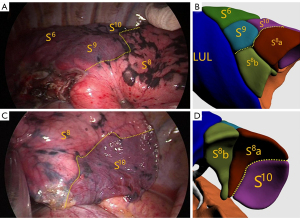
Statistical analyses
SPSS 23.0 software (IBM Corp., Armonk, NY, USA) was used for data analysis. Continuously distributed data are expressed as mean ± standard deviation, and between-group differences were identified with the Student’s t-test. Categorical variables are expressed as absolute frequencies and proportions (%). The χ2 or Fisher’s exact test was used to identify significant differences in categorical variables.
Results
General characteristics
A total of 120 eligible patients were included in the study, including 61 in the ISP group and 59 in the non-ISP group. A total of 139 nodules were removed, including 72 in the ISP group and 67 in the non-ISP group. Data on patient sex, age, maximum lesion diameter, preoperative smoking, pulmonary function, number of nodules removed, and pathological types are summarized in Table 1. There were no significant differences between the two groups in these parameters (P>0.05 for all).
Table 1
| Characteristics | ISP group (n=61) | non-ISP group (n=59) | t/χ2 | P |
|---|---|---|---|---|
| Sex, n (%) | 0.996 | 0.318 | ||
| Male | 25 (41.0) | 19 (32.2) | ||
| Female | 36 (59.0) | 40 (67.8) | ||
| Age (years) | 52.3±10.9 | 53.0±11.6 | −0.330 | 0.742 |
| Smoking, n (%) | 0.414 | 0.559 | ||
| Yes | 8 (13.1) | 5 (8.5) | ||
| No | 53 (86.9) | 54 (91.5) | ||
| Maximum lesion diameter(cm) | 0.85±0.37 | 0.89±0.37 | 0.599 | 0.550 |
| Pulmonary function | ||||
| FEV1 (L) | 2.67±0.64 | 2.45±0.69 | 1.872 | 0.064 |
| Pre-FEV1/FEV1 (%) | 92.4±14.1 | 94.3±17.0 | −0.690 | 0.492 |
| Nodules removed per case*, n (%) | 0.035 | 0.982 | ||
| 1 | 51 (83.6) | 50 (84.7) | ||
| 2 | 9 (14.8) | 8 (13.6) | ||
| 3 | 1 (1.6) | 1 (1.7) | ||
| Pathological types**, n (%) | 0.698 | 0.431 | ||
| Microinvasive adenocarcinoma | 57 (79.2) | 49 (73.1) | ||
| Invasive adenocarcinoma | 15 (20.8) | 18 (26.9) |
*, number of nodules removed per patient; **, pathological type of resected nodules. ISP, intersegmental plane.
Operation characteristics and postoperative recovery
All operations were single-port thoracic surgery. There were no cases of transfer to thoracotomy or postoperative death. The resection range in the two groups is shown in Table 2. We divided the resection range into segment, segment + segment, segment + subsegment, subsegment + subsegment, and subsegment + sub-subsegment according to different segmental combinations. The statistical analysis showed no significant difference between the two groups. All target lesions were resected in both groups. There were five cases of gross margin insufficiency in the study population, all of which were in the non-ISP group. Operation characteristics and postoperative recovery in both groups are shown in Table 3. The preoperatively simulated ISP and the actual ISP were consistent in 56 of 61 cases (91.8%).
Table 2
| Surgical range | Group ISP (n=61) | Group non-ISP (n=59) | Total |
|---|---|---|---|
| Left lung | 32 | 30 | 62 |
| S1+2 | 7 | 3 | 10 |
| S1+2 + S3a | 1 | 0 | 1 |
| S1+2a+b | 2 | 6 | 8 |
| S1+2a+b + S3b+c | 1 | 0 | 1 |
| S1+2a+b + S3c | 1 | 0 | 1 |
| S1+2a + S3c | 1 | 0 | 1 |
| S1+2c | 0 | 2 | 2 |
| S1+2c + S3a | 1 | 0 | 1 |
| S1+2c + S3ai + S4a | 1 | 0 | 1 |
| S1+2 + S3 | 1 | 2 | 3 |
| S3 | 4 | 3 | 7 |
| S3b | 1 | 0 | 1 |
| S3b+c | 2 | 0 | 2 |
| S3b + S4b | 1 | 0 | 1 |
| S3 + S4b | 0 | 1 | 1 |
| S4 + S5 | 1 | 2 | 3 |
| S4 + S5 + S1+2c | 1 | 0 | 1 |
| S4 + S5 + S1+2c + S3a | 1 | 1 | 2 |
| S6 | 0 | 6 | 6 |
| S6b + S8a | 1 | 0 | 1 |
| S6b + S8a + S* | 0 | 1 | 1 |
| S8 | 2 | 2 | 4 |
| S10 | 2 | 1 | 3 |
| Right lung | 29 | 29 | 58 |
| S1 | 3 | 5 | 8 |
| S1 + S2a | 2 | 0 | 2 |
| S1 + S2 | 0 | 1 | 1 |
| S1 + S3 | 1 | 1 | 2 |
| S1 + S3a | 1 | 0 | 1 |
| S1a | 0 | 1 | 1 |
| S1a + S2a | 2 | 1 | 3 |
| S1b + S3b | 0 | 1 | 1 |
| S1b + S3b + S2a | 0 | 1 | 1 |
| S2 | 5 | 3 | 8 |
| S2 + S1a | 2 | 1 | 3 |
| S2 + S3a | 2 | 0 | 2 |
| S2b + S3a | 1 | 0 | 1 |
| S2b + S3a + S1ai | 1 | 0 | 1 |
| S3 | 5 | 1 | 6 |
| S3 + S1b | 1 | 1 | 2 |
| S3 + S2b | 1 | 1 | 2 |
| S6 | 0 | 5 | 5 |
| S6b + S7b | 0 | 1 | 1 |
| S7 + S8 | 0 | 1 | 1 |
| S8 | 1 | 2 | 3 |
| S8a + S9 + S10 | 0 | 1 | 1 |
| S9 | 0 | 1 | 1 |
| S9 + S10 | 1 | 0 | 1 |
ISP, intersegmental plane.
Table 3
| Characteristics | ISP group (n=61) | Non-ISP group (n=59) | t/χ2 | P |
|---|---|---|---|---|
| Surgical range | 5.616 | 0.335 | ||
| Segment | 29 (47.5) | 32 (54.2) | ||
| Subsegment | 1 (1.6) | 3 (5.1) | ||
| Segment + segment | 4 (6.6) | 7 (11.9) | ||
| Segment + subsegment | 12 (19.7) | 6 (10.2) | ||
| Subsegment + subsegment | 13 (21.3) | 11 (18.6) | ||
| Subsegment + sub-subsegment | 2 (3.3) | 0 | ||
| Operation time (min) | 162.0±62.1 | 153.2±49.0 | 0.885 | 0.378 |
| Mediastinal lymph node | 0.527 | 0.468 | ||
| Dissection | 15 (24.6) | 18 (30.5) | ||
| Sampling | 46 (75.4) | 41 (69.5) | ||
| Gross margin sufficiency | 5.349 | 0.026 | ||
| Yes | 61 (100.0) | 54 (91.5) | ||
| No | 0 | 5 (8.5) | ||
| Intraoperative blood loss (mL) | 51.7±32.3 | 49.7±61.9 | 0.227 | 0.821 |
| Duration of chest drainage (days)* | 2.66±1.48 | 2.08±0.87 | 2.693 | 0.008 |
| Postoperative complications | 7 (11.5) | 12 (20.3) | 1.768 | 0.184 |
| Pulmonary infection | 4 (6.6) | 10 (16.9) | ||
| Pulmonary leakage >7 days | 1 (1.6) | 0 | ||
| Atrial fibrillation | 1 (1.6) | 2 (3.4) | ||
| Pulmonary embolism | 1 (1.6) | 0 | ||
| Postoperative hospital stay (days) | 4.5±1.9 | 4.3±1.5 | 0.468 | 0.642 |
*, two chest tubes were routinely placed during the operation. The first was a 24- to 28-French tube, which was placed at the top of the chest through the incision and connected to a water-sealed bottle for exhaust. This tube was removed when there was no air leakage. The duration of chest drainage refers to this tube. The second was an 8-French drainage tube (Disposable Abdomen Drainage Catheter Set, Guangdong Baihe Medical Technology Co., Ltd., Guangdong Province, China), which was placed at the seventh intercostal space on the midaxillary line and externally connected to the drainage bag. This tube was removed on the day of hospital discharge. ISP, intersegmental plane.
Results of 3D imaging reconstruction in the ISP group
With the IQQA-3D imaging system, a total of 131 intersegmental veins were used to evaluate the ISP in 61 patients, with an average of 2.1±0.5 veins per patient. Among these patients, two (3.3%) had one vein that could be used to evaluate the ISP, 50 (82.3%) had two, seven (11.3%) had three, and two (3.3%) had four. The total number of intersegmental veins located on the simulated ISP was 124 (94.7%), with an average of 2.0±0.6 veins per patient. The accuracy of ISP simulation was 91.8% (56/61).
Discussion
Existing problems in 3D preoperative planning
3D imaging systems provide noninvasive preoperative planning with excellent reproducibility and can provide reliable surgical plans for almost all types of segmentectomy, especially in complex cases. Use of these systems may become a main method for preoperative planning of segmentectomy. At present, various 3D reconstruction systems are used in surgical planning; however, these systems still have obvious limitations in ISP delineation. For early lung cancer, if the ISP can be accurately simulated, we can confirm preoperatively whether the planned segmentectomy meets the requirement of adequate surgical margins. Thus far, there are few reports of a good preoperative ISP simulation process. Here, we introduced an accurate preoperative ISP simulation process that could meet clinical needs. The key principles to preoperative planning are localization of target nodules and ISP simulation to facilitate complete tumor resection with sufficient surgical margins.
Accurate nodule localization
According to NCCN guidelines regarding surgical treatment of early lung cancer, complete lesion resection and sufficient surgical margins are needed; that is, parenchymal resection margins of ≥2 cm or ≥ tumor diameter. Therefore, lesion localization should include two aspects: localization of the lesion and delineation of the surgical margin. At present, multiple software packages allow lesion localization, but there is no optimal solution to delineate surgical margins. Surgical margins should be determined based on 360° 3D analysis. In our study, in which the nodule was used as the center of gravity, a spherical boundary with a margin of ≥2 cm or ≥ nodule diameter was established using the nodular analysis function of the IQQA-3D system. The spherical boundary was displayed on the 3D image so that the minimum resection range could be observed by rotating the 3D image.
Accurate simulation and evaluation of ISP
There are three major structures that define the ISP, namely the segmental bronchus, the segmental artery, and the intersegmental vein. At present, there are three main ISP delineation methods. First, intersegmental veins are identified based on the clinician’s experience and manually connected to determine the ISP. Second, the ISP is roughly delineated by scope. Third, computer-based ISP delineation based on the characteristics of the segmental bronchus, such as its course, size, and shape, is performed. In most 3D imaging systems, such as the lung operation 3D imaging system (7) (Xiamen TRONG Technology Company, Xiamen, Fujian, China), OsiriX software (8) (Pixmeo SARL, Geneva, Switzerland), and Deepinsight software (10) (First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China), the ISP is mainly determined using the first two approaches. The disadvantage of these methods is that they are greatly operator-dependent, not very reproducible, and may be inaccurate or even wrong if the clinician misunderstands or erroneously identifies intersegmental veins. Such mistakes may lead to errors in preoperative planning. Unlike other 3D imaging systems, the IQQA-3D system simulates the ISP according to the characteristics of the segmental bronchus, which has the advantage of high repeatability and is not operator-dependent (6).
There were no statistically significant differences in general characteristics between the two groups in the present study. In terms of postoperative recovery, there was a significant difference between the two groups in the duration of chest drainage, but there was no significant difference in the length of postoperative hospital stay. In the comparison of operation characteristics, we found a statistically significant difference between the two groups in the rate of gross margin insufficiency. Five patients in the non-ISP group underwent wedge resection because of an insufficient margin. The bronchus-vein-artery triad method, which ensures accurate ISP simulation to facilitate complete tumor resection with sufficient surgical margins, was applied to reduce the rate of margin insufficiency.
Bronchus-vein-artery triad
The ISP was delineated and evaluated using the bronchus, pulmonary artery, and pulmonary vein, or the bronchus-vein-artery triad, as follows.
Segmental bronchus: in the IQQA-3D system, ISP simulation was performed based on the characteristics of the bronchus.
Intersegmental vein
The ISP is often an irregular plane that cannot be completely represented by the linear intersegmental vein; therefore, ISP delineation using the intersegmental vein (the first ISP delineation method outlined above) is not sufficiently accurate. In our study, ISP simulation was not related to the intersegmental vein, but to the segmental bronchi. In this case, when the simulated intersegmental vein fell on the simulated ISP, the simulated ISP was deemed accurate and acceptable (Figure 5). This method can be used to verify the accuracy of ISP simulation. If the intersegmental vein does not fall on the simulated ISP, this indicates that there are errors in the simulated ISP. The influence of these errors should be considered during surgery. In this study, 94.7% of intersegmental veins (124/131) fell on the simulated ISP, which is consistent with intraoperative findings, indicating that ISP delineation based on the bronchus was consistent with the actual ISP. We found that 5.3% of intersegmental veins (7/131) did not fall on the simulated ISP; the quality of CT images in these cases was poor, resulting in inaccurate tracheal reconstruction and partial lack of distal bronchial reconstruction, which were likely the main reasons for inaccurate ISP simulation (Figure 6).
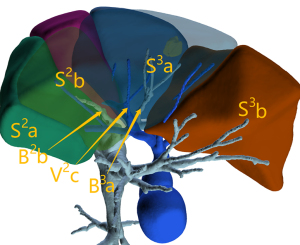
Segmental artery
Sun et al. (12) compared the infrared indocyanine green method with the inflation-deflation method of locating the ISP and confirmed that the two methods were completely concordant. Even specific details, such as an irregular convex surface, matched completely. The former method shows the ISP according to the reverse staining method; the target segment is revealed by lack of blood flow caused by pulmonary artery dissection. The theory behind the inflation-deflation method remains unclear. One of the most widely accepted explanations is that when the targeted segment bronchus is blocked, gaseous exchange between the targeted segment and the atmosphere cannot take place. At the same time, Kohn’s pores do not open, which leads to continuous expansion of the targeted segment (13-15). Sun et al. showed that the ISP revealed with the inflation-deflation method was determined by the target pulmonary artery. In our study, 56 of 61 patients (91.8%) in the ISP group had an ISP that was consistent with preoperative ISP simulation. The simulated intersegmental veins fell on the simulated ISP in all 56 cases, which indicates that ISP simulation based on segmental bronchi is consistent with intraoperative ISP determination based on segmental arteries.
According to the bronchus-vein-artery triad, intersegmental veins located on the ISP indicate the accuracy of ISP simulation. The preoperative ISP simulated on the basis of target bronchi was consistent with the intraoperative ISP based on the target segmental artery. Preoperative ISP delineation was consistent with intraoperative findings. In this study, the peripheral bronchi were not clear on CT images in five patients (8.2%), resulting in incomplete bronchi in the 3D imaging system. These cases were inconsistent, mainly because of ISP simulation errors caused by bronchial reconstruction errors. We adjusted the resection range to ensure adequate surgical margins; when the simulated intersegmental vein fell within the target segment and the safe resection margin sphere exceeded the intersegmental vein, we increased the wedge resection of the adjacent segment to ensure adequate surgical margins. When the simulated intersegmental vein fell within a non-target segment, we performed resection according to the intraoperative ISP.
Advantages of the bronchus-vein-artery triad
The bronchus-vein-artery triad offers several benefits. First, it facilitates accurate ISP delineation, which is important in the surgical planning of segmentectomy. If the intersegmental veins fall on the ISP in the IQQA-3D system, this indicates accurate ISP delineation, which ensures agreement between surgery and preoperative planning. This simulation is helpful to facilitate complete tumor resection and to obtain sufficient surgical margins. Second, this strategy is conducive to the accurate planning of surgical margins in complex segmentectomy, preventing inadequate margins and even missed resection. Third, the bronchus-vein-artery triad can be used for ISP simulation in other 3D imaging systems, and the accuracy of ISP simulation can be evaluated in the same way.
Limitations of the bronchus-vein-artery triad
When the quality of CT images is poor, the quality of 3D reconstruction will be affected, which may lead to inaccurate ISP delineation and inappropriate preoperative planning. These errors should be considered in preoperative planning. The performance of this strategy in delineating the ISP of segments and subsegments is good; however, there were few cases of sub-subsegmental resection in this study. Determination of the ISP between sub-subsegments remains unclear and needs further exploration.
In conclusion, application of the bronchus-vein-artery triad in ISP simulation can assist surgeons in performing preoperative planning, facilitating complete resection of early lung cancer with sufficient surgical margins.
Acknowledgments
We thank Emily Woodhouse, PhD, from Liwen Bianji (Edanz), for editing the English text of a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-822
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tcr-21-822
Peer Review File: Available at https://dx.doi.org/10.21037/tcr-21-822
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-822). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the institutional review board of Fujian Medical University Union Hospital (No. 2021KY112), which waived the need for informed consent of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stamatis G, Leschber G, Schwarz B, et al. Perioperative course and quality of life in a prospective randomized multicenter phase III trial, comparing standard lobectomy versus anatomical segmentectomy in patients with non-small cell lung cancer up to 2 cm, stage IA (7th edition of TNM staging system). Lung Cancer 2019;138:19-26.
- Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- Brown LM, Louie BE, Jackson N, et al. Recurrence and Survival After Segmentectomy in Patients With Prior Lung Resection for Early-Stage Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1110-8. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Xu G, Chen C, Zheng W, et al. Application of the IQQA-3D imaging interpretation and analysis system in uniportal video-assisted thoracoscopic anatomical segmentectomy: a series study. J Thorac Dis 2019;11:2058-66. [Crossref] [PubMed]
- Yang Q, Xie B, Hu M, et al. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg 2016;23:183-9. [Crossref] [PubMed]
- Yao F, Wang J, Yao J, et al. Three-dimensional image reconstruction with free open-source OsiriX software in video-assisted thoracoscopic lobectomy and segmentectomy. Int J Surg 2017;39:16-22. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016;8:S710-5. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Thoracoscopic Pulmonary Sub-Subsegmentectomy Based on Three-Dimensional Images. Ann Thorac Surg 2016;102:e389-91. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer (Version 2019) [EB/OL]. Fort Washington: NCCN, 2019. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Sun Y, Zhang Q, Wang Z, et al. Is the near-infrared fluorescence imaging with intravenous indocyanine green method for identifying the intersegmental plane concordant with the modified inflation-deflation method in lung segmentectomy? Thorac Cancer 2019;10:2013-21. [Crossref] [PubMed]
- Van Allen CM, Lindskog GE, Richter HG. Collateral respiration. transfer of air collaterally between pulmonary lobules. J Clin Invest 1931;10:559-90. [Crossref] [PubMed]
- Cordingley JL. Pores of Kohn. Thorax 1972;27:433-41. [Crossref] [PubMed]
- Wareham EE, Huse WM. Surgical Anatomy of the Lungs. Surgical Clinics of North America 1964;44:1191-200. [Crossref]


