
The clinical prognostic factors of patients with stage IB lung adenocarcinoma
Introduction
Lung cancer is the most common malignant tumor with the highest morbidity and mortality worldwide (1). Adenocarcinoma (ADC) has been the primary subtype of lung cancer, accounting for 55% in recent years, with a strong proliferative capacity and a high degree of malignancy. Some patients have localized tumor infiltration or distant metastasis at the time of diagnosis, and the prognosis is poor (2,3).
The 8th edition of the TNM staging of the Lung Cancer, launched by the International Union Against Cancer (UICC) on January 1, 2018, had undergone numerous changes and additions compared to the 7th edition. It is now frequently used to predict the survival of patients with lung adenocarcinoma. In terms of tumor size, the 8th edition staged a more detailed classification of stage Ib tumors (3 cm < T2a ≤4 cm) (4-6). And tumor invasion of the pleural/elastic layer (PL) also belongs to stage IB, which has been reported as a poor prognostic factor in ADC (7,8). Differences and disputes still existed among patients with stage IB lung adenocarcinoma in survival status and related treatment recommendations (6,9-12). The influence of clinical factors on survival status was more or less various in studies (13-16).
SEER recently released the data of patients diagnosed with lung cancer in 2016. Therefore, the purpose of this study was to analyze the factors associated with the prognosis of patients with stage IB lung adenocarcinoma among 2010–2016, especially illustrated whether tumor size and PL play an important role or not, which may help improve the treatment strategy for early-stage lung cancer patients.
We present the following article in accordance with the TRIPOD reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-1174).
Methods
Data sources and patient cohort
The data of patients were collected from the Surveillance, Epidemiology, and End Results (SEER) public use database SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (2010–2016).
A total of 8,846 patients with complete follow-up data were diagnosed as stage IB (AJCC 8th) ADC and performed surgery between 2010 and 2016 in the SEER database. Among them, 7,605 patients were finally enrolled in cohort I.
The characteristics of these patients are reported in Table 1, which includes age at the time of subsequent cancer diagnosis, race, gender, primary site, pathological classification (histology), grade, laterality, first malignant primary indicator, total no. of malignant and benign tumors, pleural/elastic layer invasion (PL) and tumor size. Finally, 5,324 patients with stage IB ADC from the SEER database were randomly assigned to the training cohort, and 2,281 were in the test cohort.
Table 1
| Characteristics | Cohort 1 | Validation cohort | |||
|---|---|---|---|---|---|
| SEER database, n=7,605 | Our database, n=272 | ||||
| n | % | n | % | ||
| Age | |||||
| ≤60 yr | 1,181 | 15.53% | 102 | 37.50% | |
| 61–70 yr | 2,459 | 32.33% | 92 | 33.82% | |
| 71–80 yr | 2,910 | 38.26% | 71 | 26.10% | |
| >80 yr | 1,055 | 13.87% | 7 | 2.57% | |
| Race | |||||
| Black | 686 | 9.02% | 0 | 0.00% | |
| Others | 53 | 0.70% | 0 | 0.00% | |
| Asian or Pacific islander | 646 | 8.49% | 272 | 100.00% | |
| White | 6,220 | 81.79% | 0 | 0.00% | |
| Sex | |||||
| Female | 4,097 | 53.87% | 147 | 54.04% | |
| Male | 3,508 | 46.13% | 125 | 45.96% | |
| Differentiated grade | |||||
| Well differentiated | 1,241 | 16.32% | 57 | 20.96% | |
| Moderately differentiated | 3,767 | 49.53% | 155 | 56.99% | |
| Poorly differentiated | 2,465 | 32.41% | 60 | 22.06% | |
| Undifferentiated | 132 | 1.74% | 0 | 0.00% | |
| Laterality | |||||
| Right | 4,509 | 59.29% | 172 | 63.24% | |
| Left | 3,096 | 40.71% | 100 | 36.76% | |
| Surgery to the primary site | |||||
| Sublobectomy | 1,533 | 20.16% | 0 | 0.00% | |
| Multiple lobes | 933 | 12.27% | 5 | 1.84% | |
| Lobectomy | 5087 | 66.89% | 262 | 96.32% | |
| Pneumonectomy | 52 | 0.68% | 5 | 1.84% | |
| Tumor size | |||||
| ≤10 mm | 210 | 2.76% | 40 | 14.71% | |
| 11–20 mm | 1,603 | 21.08% | 112 | 41.18% | |
| 21–30 mm | 1,684 | 22.14% | 84 | 30.88% | |
| 31–35 mm | 2,557 | 33.62% | 21 | 7.72% | |
| 36–40 mm | 1,551 | 20.39% | 15 | 5.51% | |
| Pleural/Elastic Layer Invasion (PL) | |||||
| PL=0, No evidence of PL invasion | 4,149 | 54.56% | 78 | 28.68% | |
| PL=1, Invasion beyond the visceral elastic pleura, but limited to the pulmonary pleura | 1,995 | 26.23% | 160 | 58.82% | |
| PL=2, Invasion to the surface of the pulmonary pleura | 1,461 | 19.21% | 34 | 12.50% | |
| Tumor size & PL | |||||
| ≤30 mm, PL=1 or 2 | 3,497 | 45.98% | 236 | 86.76% | |
| 31–40 mm, PL=0 | 3,369 | 44.30% | 12 | 4.41% | |
| 31–40 mm, PL=1 or 2 | 739 | 9.72% | 24 | 8.82% | |
A total of 272 ADC at stage IB patients performed surgery for primary ADC lesion in the Department of Thoracic Surgery of Zhongshan Hospital Affiliated to Fudan University (ZHTS) were included. The selection process is shown in Figure 1.
Statistical analysis
The distribution of patients’ characteristics (gender, race, age, primary site, pathological classification, differentiation grade, and chemotherapy, etc.) was summarized using counts and percentages. Statistical analysis was done using R Project (https://www.r-project.org) and SPSS 23.0 software (IBM). Kaplan-Meier method was used for the survival analysis. Multivariate survival analysis was calculated by the Cox proportional hazards regression. The test level was α=0.05, and the difference was statistically significant at P<0.05.
The prognostic model was then used to predict the 3-year outcomes of OS. We validated the nomogram internally and externally both in the training group and in the validation group. Harrell Consistency Index (C-Index) were used to evaluate the nomogram, with a higher C-index indicating a more accurate prognostic predictions (17). The calibration plot was adopted to evaluate nomogram performance. The C-index, nomogram, calibration curves and Kaplan-Meier curves were generated in R with packages “rms”, “survival”, “foreign” and “regplot” respectively (18).
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committees of Zhongshan Hospital, Fudan University (Shanghai, China) (Approval No.: B2019-232R). Informed consent forms were exempt.
Results
Patient characteristics
Among stage IB patients, the predominant age group was 71–80 years in the SEER database, while ≤60 years was the majority in the validation cohort. For the differentiated grade, the vast majority was moderately differentiated in all databases. Most of the patients enrolled in our study were performed surgery with Lobectomy + LN dissection. Details were described in Table 1.
Survival time analysis
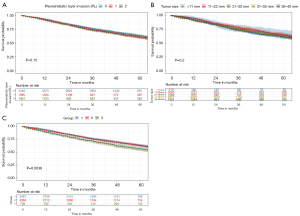
A Kaplan-Meier analysis was conducted to evaluate the cumulative risk for the development of stage IB lung and was illustrated in Figure 2. The risk for development of stage IB lung cancer was neither related to PL (PL=0, PL=1, PL=2, P=0.15, Figure 2A), nor the tumor size (≤1 cm, 1.1–2.0 cm, 2.1–3.0 cm, 3.1–4.0 cm, P=0.2, Figure 2B) alone. However, once tumor size was considered in combination with PL, patients with stage IB lung cancer showed a significantly different survival status (P=0.0038, Figure 2C).
Cox survival analysis
Univariate analysis (Table 2) revealed that age at diagnosed (P<0.001), race (P<0.001), sex (P<0.001), tumor differentiation grade (P<0.001), total no. of in situ/malignant tumors for the patient (P<0.001), surgery to the primary site (P<0.001), group (P=0.005) was significant predictors of stage IB lung cancer patients. Multivariate Cox proportional hazard analysis of all IB staged patients (Table 2) demonstrated sex (P<0.001), age (P<0.001), race (P=0.003), tumor differentiation grade (P<0.001), surgery to the primary site (P<0.001), group (P<0.001), were independent prognostic factors for better survival in the IB staged patients (AJCC 8th). No significant difference was caused by tumor size or total no. of in malignant tumors for patient.
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age at diagnosed | |||||||
| ≤60 yr | reference | reference | |||||
| 61–70 yr | 0.480 | 0.414–0.558 | < 0.001 | 1.311 | 1.120–1.534 | 0.001 | |
| 71–80 yr | 1.372 | 1.225–1.537 | < 0.001 | 1.889 | 1.625–2.196 | <0.001 | |
| >80 yr | 0.684 | 0.617–0.758 | < 0.001 | 2.470 | 2.091–2.919 | <0.001 | |
| Race | < 0.001* | 0.003 | |||||
| White | reference | reference | |||||
| Others | 1.508 | 0.705–3.225 | 0.290 | 0.501 | 0.238–1.053 | 0.068 | |
| Asian or Pacific islander | 1.889 | 0.888–4.016 | 0.098 | 0.742 | 0.621–0.886 | 0.001 | |
| Black | 2.139 | 1.018–4.493 | 0.045 | 0.969 | 0.834–1.126 | 0.682 | |
| Sex | <0.001* | <0.001* | |||||
| Female | reference | reference | |||||
| Male | 1.385 | 1.273–1.506 | <0.001 | 1.326 | 1.218–1.443 | <0.001 | |
| Differentiated Grade | <0.001* | <0.001* | |||||
| Well differentiated | reference | reference | |||||
| Moderately differentiated | 0.560 | 0.409–0.767 | <0.001 | 1.432 | 1.246–1.645 | <0.001 | |
| Poorly differentiated | 0.837 | 0.623–1.125 | 0.239 | 1.853 | 1.608–2.135 | <0.001 | |
| Undifferentiated | 1.100 | 0.818–1.480 | 0.528 | 1.869 | 1.364–2.560 | <0.001 | |
| Laterality | 0.169 | Not included | |||||
| Right | reference | ||||||
| Left | 1.062 | 0.975–1.156 | 0.169 | ||||
| Surgery to the primary site | <0.001* | <0.001* | |||||
| Sublobectomy | reference | reference | |||||
| Multiple lobes | 0.732 | 0.640–4.450 | <0.001 | 0.839 | 0.727–0.969 | 0.017 | |
| Lobectomy + LN dissection | 0.555 | 0.504–0.612 | <0.001 | 0.684 | 0.605–0.773 | <0.001 | |
| Pneumonectomy | 0.528 | 0.197–1.413 | <0.001 | 0.698 | 0.260–1.872 | 0.474 | |
| Tumor size | 0.197 | Not included | |||||
| ≤10 mm | reference | ||||||
| 11–20 mm | 0.900 | 0.690–1.172 | 0.433 | ||||
| 21–30 mm | 0.856 | 0.750–0.976 | 0.020 | ||||
| 31–35 mm | 0.957 | 0.843–1.087 | 0.500 | ||||
| 35–40 mm | 0.953 | 0.849–1.069 | 0.409 | ||||
| Pleural/Elastic Layer Invasion (PL) | 0.154 | Not included | |||||
| No evidence of PL invasion | reference | ||||||
| Invasion beyond the visceral elastic pleura, but limited to the pulmonary pleura | 1.074 | 0.971–1.188 | 0.164 | ||||
| Invasion to the surface of the pulmonary pleura | 1.101 | 0.985–1.230 | 0.090 | ||||
| Tumor size & PL (group) | 0.005 | 0.964 | 0.868–1.071 | <0.001 | |||
| ≤30 mm | reference | reference | |||||
| 31–40 mm, PL=0 | 1.032 | 0.944–1.128 | 0.494 | 1.145 | 1.043–1.256 | 0.004 | |
| 31–40 mm, PL=1 or 2 | 1.269 | 1.101–1.463 | 0.001 | 1.327 | 1.149–1.532 | <0.001 | |
*, indicate a statistical significance.
Contribution and validations of the nomogram
A nomogram relating to 6 independent risk factors (age, race, sex, tumor histological, grade, surgery, and group), which were concluded from MVA (Figure 3). 3-year overall survival (OS) could be calculated by the Points at the top of the model (Figure 3A). The internal evaluation was performed (Figure 3B) as well as the external evaluation (Figure 3C) with the same database. The C-indexes for 3-year OS were 0.644±0.015 (training cohort, SEER database) and 0.625±0.024 (test cohort, SEER database).
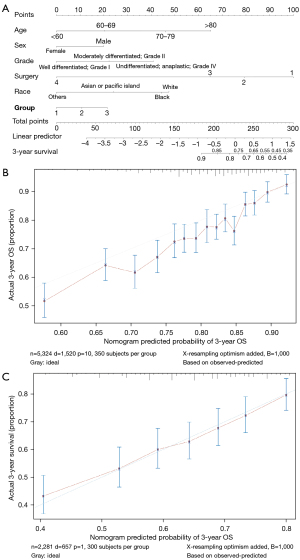
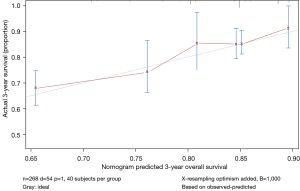
Furthermore, we verified our nomogram model by individuals with entirely different characteristics of the data (Figure 4), the C-index of which was 0.690±0.079 (database in our department).
In general, IB ADC patients who had a younger age, female sex, non-black-or-white race, lower differentiated level or performed pneumonectomy had longer predicting survival time. For the groups, those in group 1, which meant the tumor size was less than 30 mm had the best clinical outcomes, followed by 31–40 mm tumor size with no PL invasion, and those with 31–40 mm tumor size with PL invasion behaved worst in survival time.
Discussion
In our study, we found that in patients with stage IB ADC, the differences in tumor size or PL invasion didn’t cause differences in living conditions, while the survival times appeared different once both of them were considered together. In all, six independent risk factors (age, race, sex, tumor histological, grade, surgery, and group) were concluded from MVA and contributed for nomogram model. Recently, study considering stage IB NSCLC concluding similar independent risk factors, including age, sex, histology, tumor differentiation (19), and it was widely proved that among lung cancer patients, female patients have a better prognosis (20), which was also revealed in our research.
The pleural invasion was well-positioned as a T2 descriptor and led to a worse prognosis even after adjusting for the current tumor size cut points (21-25). Our result was similar to the research result that IB patients with both pleural invasion and tumor size between 3.1–4.0 cm had a closer survival status to the stage IIA patients (14). Rami-Porta’s study also suggested that 3-cm cutoff point still separates T1 from T2 tumors, but tumor size arises as a more important prognostic factor, because, from ≤1 to 5 cm, each centimeter separates tumors with a significantly different prognosis (21), while Nitadori et al. found that PL distinguished OS in patients with lung adenocarcinoma with a tumor size of 2–3 cm, but failed to stratify patients with a tumor size of ≤2 cm (26). Other researchers showed that the presence of PL, not the depth of invasion, was associated with postoperative survival (23,27,28), but conflicted to the conclusion that survival differences existed among different PL stages (29,30). More studies can be focused on this phenomenon to illustrate the probable mechanism.
In addition to the tumor size and the degree of local invasion, for patients with stage IB lung adenocarcinoma, men, blacks, whites, etc., are related to poorer prognosis, so they are more likely to require further treatment. In addition, patients undergoing sublobectomy and multiple lobectomy also have a poorer prognosis, which may be related to the failure of complete removing of the lesion. Therefore, the follow-up after the operation should be more closely to better determine whether it is necessary to apply further treatment. In conclusion, those results indicate we should take different clinical decisions for different patients, even if they have the same clinical stage.
Furthermore, FDG-PET/CT SUVmax, the value of which reflects the biological activity of tumors, is also closely related to tumor proliferation, invasion, progression and metastasis (31). Kawakita/Toba reported that FDG-PET/CT SUVmax, total tumor size, and could predict the prognosis of pStage I lung adenocarcinoma based on the 7th edition of the TNM staging system (32). it was also found that solid predominant types have high SUVmax values and a shorter PFS than the other histologic subtypes (33).
Recently, the therapy strategy for IB lung cancer patients had been widely discussed. The recent National Comprehensive Cancer Network (NCCN) guidelines stated that adjuvant chemotherapy could be used for patients with stage IB NSCLC having high-risk factors including poorly differentiated tumors, vascular invasion, wedge resection, tumors >4 cm, visceral pleural involvement, and unknown lymph node status (Nx), which independently may not be an indication and may be considered when determining treatment with adjuvant chemotherapy (34). NSCLC Meta-analysis Collaborative Group’s meta-analysis (35), mainly on stage IB–IIIA patients, achieved the conclusion that preoperative chemotherapy significantly improves overall survival in resectable NSCLC and some other studies reached the similar conclusion that adjuvant chemotherapy may improve the OS of completely resected patients with a solid predominant tumor pattern in stage IB ADC (36,37). In contrast, there were also studies that showed that adjuvant chemotherapy was associated with worse OS than observation or no significant survival advantage for patients with stage IB NSCLC, but with significant OS benefit in stage IIA setting based on the 8th edition staging (6,9).
According to our research, visceral pleural involvement was not an independent prognostic factor in patients with stage IB lung cancer based on the 8th editions of AJCC TNM staging system. To decide whether patients should be treated with adjuvant chemotherapy, both tumor size and PL can be considered.
The limitation of this study is that, firstly, because the SEER database used in this study has no chemotherapy-related records for lung cancer patients diagnosed in 2016, it is unable to conduct further statistical analysis on lung cancer treatment. Since the SEER database is predominantly white, certain biases will be introduced when analyzing the impact of race on the prognosis, and further research is needed to explore whether race is really a factor influencing the prognosis of lung cancer. Furthermore, the patients’ detailed clinical information is limited in the SEER database, as there is no record of PET/CT SUVmax value and other prognosis-related figures for the further analyze. In addition, this study is only a retrospective study, and further experiments are needed to verify or clarify the relevant conclusions.
Conclusions
The combination of tumor size and PL invasion is a significant clinical character of different prognosis in patients with stage IB lung adenocarcinoma (AJCC 8th TNM classification), which may help the selection of patients who might benefit from chemotherapy and more advanced treatment.
Acknowledgments
The authors thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-1174
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tcr-21-1174
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-1174). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committees of Zhongshan Hospital, Fudan University (Shanghai, China) (Approval No.: B2019-232R). Informed consent forms were exempt.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Yoon JY, Sigel K, Martin J, et al. Evaluation of the Prognostic Significance of TNM Staging Guidelines in Lung Carcinoid Tumors. J Thorac Oncol 2019;14:184-92. [Crossref] [PubMed]
- Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res 2019;11:943-53. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Detterbeck FC, Chansky K, Groome P, et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1433-46. [Crossref] [PubMed]
- Wang J, Wu N, Lv C, et al. Should patients with stage IB non-small cell lung cancer receive adjuvant chemotherapy? A comparison of survival between the 8th and 7th editions of the AJCC TNM staging system for stage IB patients. J Cancer Res Clin Oncol 2019;145:463-9.
- Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;130:160-5. [Crossref] [PubMed]
- Lin W, Huang M, Zhang Z, et al. A retrospective study of the relationship between the pathologic subtype and lymph node metastasis of lung adenocarcinomas of ≤3 cm diameter. Medicine (Baltimore) 2020;99:e21453. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Park HJ, Park HS, Cha YJ, et al. Efficacy of adjuvant chemotherapy for completely resected stage IB non-small cell lung cancer: a retrospective study. J Thorac Dis 2018;10:2279-87. [Crossref] [PubMed]
- Nakajima K, Iwata H, Ogino H, et al. Clinical outcomes of image-guided proton therapy for histologically confirmed stage I non-small cell lung cancer. Radiat Oncol 2018;13:199. [Crossref] [PubMed]
- Liu Y, Shan L, Shen J, et al. Choice of surgical procedure - lobectomy, segmentectomy, or wedge resection - for patients with stage T1-2N0M0 small cell lung cancer: A population-based study. Thorac Cancer 2019;10:593-600. [Crossref] [PubMed]
- Zeng Y, Mayne N, Yang CJ, et al. A Nomogram for Predicting Cancer-Specific Survival of TNM 8th Edition Stage I Non-small-cell Lung Cancer. Ann Surg Oncol 2019;26:2053-62.
- Yang X, Sun F, Chen L, et al. Prognostic value of visceral pleural invasion in non-small cell lung cancer: A propensity score matching study based on the SEER registry. J Surg Oncol 2017;116:398-406. [Crossref] [PubMed]
- Abdel-Rahman O. Challenging a dogma; AJCC 8th staging system is not sufficient to predict outcomes of patients with malignant pleural mesothelioma. Lung Cancer 2017;113:128-33. [Crossref] [PubMed]
- Miura K, Hamanaka K, Koizumi T, et al. Solid component tumor doubling time is a prognostic factor in non-small cell lung cancer patients. J Cardiothorac Surg 2019;14:57. [Crossref] [PubMed]
- Huitzil-Melendez FD, Capanu M, O’Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 2010;28:2889-95. [Crossref] [PubMed]
- Li Y, Ma L. Nomograms predict survival of patients with lymph node-positive, luminal a breast cancer. BMC Cancer 2021;21:965. [Crossref] [PubMed]
- Zuo Z, Zhang G, Song P, et al. Survival Nomogram for Stage IB Non-Small-Cell Lung Cancer Patients, Based on the SEER Database and an External Validation Cohort. Ann Surg Oncol 2021;28:3941-50. [Crossref] [PubMed]
- Xie J, Zhang X, Hu S, et al. Effects of adjuvant chemotherapy on survival of patients with stage IB non-small cell lung cancer with visceral pleural invasion. J Cancer Res Clin Oncol 2020;146:2231-9. [Crossref] [PubMed]
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Tao H, Hayashi T, Sano F, et al. Prognostic impact of lymphovascular invasion compared with that of visceral pleural invasion in patients with pN0 non-small-cell lung cancer and a tumor diameter of 2 cm or smaller. J Surg Res 2013;185:250-4. [Crossref] [PubMed]
- Kudo Y, Saji H, Shimada Y, et al. Impact of visceral pleural invasion on the survival of patients with non-small cell lung cancer. Lung Cancer 2012;78:153-60. [Crossref] [PubMed]
- Lakha S, Gomez JE, Flores RM, et al. Prognostic significance of visceral pleural involvement in early-stage lung cancer. Chest 2014;146:1619-26. [Crossref] [PubMed]
- Neri S, Yoshida J, Ishii G, et al. Prognostic impact of microscopic vessel invasion and visceral pleural invasion in non-small cell lung cancer: a retrospective analysis of 2657 patients. Ann Surg 2014;260:383-8. [Crossref] [PubMed]
- Nitadori JI, Colovos C, Kadota K, et al. Visceral pleural invasion does not affect recurrence or overall survival among patients with lung adenocarcinoma ≤ 2 cm: a proposal to reclassify T1 lung adenocarcinoma. Chest 2013;144:1622-31. [Crossref] [PubMed]
- Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1384-90.
- Adachi H, Tsuboi M, Nishii T, et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;48:691-7; discussion 697. [Crossref] [PubMed]
- Wang T, Zhou C, Zhou Q. Extent of Visceral Pleural Invasion Affects Prognosis of Resected Non-small Cell Lung Cancer: A meta-analysis. Sci Rep 2017;7:1527. [Crossref] [PubMed]
- Liu QX, Deng XF, Zhou D, et al. Visceral pleural invasion impacts the prognosis of non-small cell lung cancer: A meta-analysis. Eur J Surg Oncol 2016;42:1707-13. [Crossref] [PubMed]
- Takenaka T, Yano T, Ito K, et al. Biological significance of the maximum standardized uptake values on positron emission tomography in non-small cell lung cancer. J Surg Oncol 2009;100:688-92. [Crossref] [PubMed]
- Kawakita N, Toba H, Kawakami Y, et al. Use of a prognostic risk score that aggregates the FDG-PET/CT SUVmax, tumor size, and histologic group for predicting the prognosis of pStage I lung adenocarcinoma. Int J Clin Oncol 2020;25:1079-89. [Crossref] [PubMed]
- Ercelep O, Alan O, Telli TA, et al. Differences in PET/CT standardized uptake values involvement and survival compared to histologic subtypes of lung adenocarcinoma. Tumori 2021;107:231-7. [PubMed]
- NCCN Clinical Practice Guidelines in Oncology-Non–Small Cell Lung Cancer. In: Version 4. 2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 2019-04-29.
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Cao S, Teng J, Xu J, et al. Value of adjuvant chemotherapy in patients with resected stage IB solid predominant and solid non-predominant lung adenocarcinoma. Thorac Cancer 2019;10:249-55. [Crossref] [PubMed]
- Qian F, Yang W, Wang R, et al. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J Thorac Cardiovasc Surg 2018;155:1227-1235.e2. [Crossref] [PubMed]



