
Tumor-associated eosinophilia in a patient with EGFR-positive, MET-amplified lung adenocarcinoma refractory to targeted therapy: a case report and review of literature
Introduction
Occurring in approximately 1% of patients, paraneoplastic eosinophilia is a rare phenomenon in solid tumors (1). Eosinophilia refers to an increase in the number of eosinophils, which are granulocytic leukocytes normally formed in the bone marrow (BM). Upon maturation or in response to multiple stimuli, eosinophils enter circulation and migrate to destination tissues (2). Eosinophilia can occur in the peripheral blood (blood eosinophilia) or tissue (tissue eosinophilia). Blood eosinophilia is graded as mild with an absolute eosinophil count (AEC) of 0.5–1.5×109/L, moderate (1.5–5.0×109/L), and severe (>5.0×109/L). The causes are manifold and therefore identified by process of exclusion. The common ones include hematological malignancies, helminth infection, allergic reaction, autoimmune conditions, and tumor necrosis or extensive metastasis (3). In the English literature there are over 20 cases of blood eosinophilia associated with primary non-small cell lung cancer (NSCLC) prior to treatment (Table 1). All but two of these patients were male and the most had adenocarcinoma or large cell carcinoma. Eosinophilia is clinically important in that it could induce damage in multiple end organs and impair the functional status of the patient, thereby limiting anticancer therapy (2). Treatment with steroid or hydroxyurea provides short-term symptomatic relief, although management of the underlying malignancy is regarded as the mainstay (10-13,15). There is also evidence of blood eosinophilia as a potential poor prognosticator or a predictor of favorable response to immunotherapy (23-26). Herein, we describe a 64-year-old male smoker with metastatic lung adenocarcinoma associated with blood eosinophilia.
Table 1
| Reference | Age (yrs) | Sex | Ever-smoker | Subtype | Pathologic stage | Blood AEC (109/L) at diagnosis | BM EOS at diagnosis (%) | Initial treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| (4) | 71 | M | N | LCC | NR (was resectable) | 5 | NR | Resection | Eosinophilia resolved after tumor resection but recurred three months later. Imaging revealed new masses. Corticosteroid achieved drastic yet transient drop in AEC. Patient died after refusing further therapy |
| (5) | 52 | M | Y | LCC | NR (was resectable) | 1.1 | NR | Resection | Eosinophilia resolved after tumor resection but recurred 8 months later, one month after which a metastasis appeared, followed by pleural effusion and worsening symptoms. Eosinophilia increased rapidly. Patient died 16 months after diagnosis |
| (6) | 55 | M | Y | SCC | NR (patient refused surgery) | 0.9 | 13.2 | Chemotherapy | Eosinophilia resolved after chemotherapy started, accompanied by reduction in tumor size and pleural effusion before patient was lost to further follow-up |
| (7) | 65 | M | Y | SCC | NR | 30.8 | Prominent eosinophilia | NR | NR |
| (7) | 68 | M | N | ADC | NR | 114.4 | NR | NR | NR |
| (8) | 72 | M | NR | LCC | IIIA | 90 | Eosinophilic hyperplasia | Resection | Eosinophilia resolved after tumor resection along with elevated serum interleukin-5 |
| (9) | 53 | M | Y | LCC | IV | 14.6 | NR | Palliative chemotherapy and brain radiotherapy | Peripheral blood eosinophilia worsened six weeks after diagnosis. Patient manifested altered mental state and increase in shortness of breath 4 weeks into therapy and in died few days later |
| (10) | 82 | M | Y | ADC | NR | 23.7 | 39 | Hydroxyurea and corticosteroid | The patient’s condition worsened rapidly while blood AEC increased 4-fold. Blood eosinophilia was alleviated following treatment. Patient died within ten days after admission |
| (11) | 52 | M | NR | ADC | NR | 82.3 | Marked eosinophilia | Hydroxyurea and corticosteroid | There was only modest reduction in the eosinophilia. Patient died 47 days after admission |
| (12) | 60 | F | NR | ADC | IV | 26.2 | NR | Steroids | Eosinophilia was alleviated upon steroids treatment and re-emerged during hospice. The patient died approximately one month after diagnosis |
| (13) | 35 | M | N | ADC | NR (met suspected) | 11.3 | NR | Steroid and other symptomatic therapy | Blood eosinophilia did not respond to treatment. Patient was started on chemotherapy and discharged on request after one cycle |
| (14) | 55 | M | Y | ADC | IV | 27.4 | NR | Palliative radiation | The patient completed 13 sessions of radiation without improvement in eosinophilia or symptoms, and was discharged to hospice |
| (15) | 54 | M | Y | ADC | NR | 3 | NR | Steroids and allopurinol | Chemotherapy after initial treatment induced marked decline in leukocytosis and eosinophilia. AEC started rising after 6 cycles when new metastases were found. Patient died 13 months after diagnosis |
| (16) | 68 | M | NR | ADC | III | 79.6 | NR | Hydroxyurea | Response was not specified but the patient was later started on chemotherapy, although his respiratory status deteriorated and the patient passed away |
| (17) | 65 | M | NR | NR | IV | 41.04 | NR | Hydroxycarbamide | Patient manifested rapidly progressing respiratory insufficiency with hypercapnia on hydroxycarbamide. Afterwards, cyclophosphamide appeared to induce drastic decrease in AEC. Comfort therapy was initiated after lung cancer was diagnosed. The patient died |
| (18) | 61 | M | Y | LCC | NR (IV suspected based on malignant pleural effusion) | 23–31 | Marked hypereosinophilia | Carboplatin plus paclitaxel | Disease progressed with recurrent massive pleural effusion and a mediastinal shift to the left side. Second-line chemotherapy was started, but the patient died due to disease progression and respiratory failure 2 months after diagnosis |
| (19) | 68 | M | NR | SCC | Recurrent | 11.2 | Marked eosinophilia | Methylprednisolone | Methylprednisolone induced rapidly decreasing AEC, but patient’s condition worsened rapidly and he died on the 5th day of hospitalization |
| (20) | 69 | M | Y | LCC | NR | 1.2 | NR | Cytoxan plus adriamycin | Respiratory difficulties and weight loss progressed over the clinical course while blood eosinophilia worsened. Chemotherapy did not result in clinical improvement. The patient died 12 months after symptom onset |
| (21) | 60 | M | Y | SCC | NR | 33.9 | NR | Prednisolone | Eosinophilia was noted on readmission 6 months after palliative pneumonectomy. Prednisolone did not lead to improved AEC. Patient died 15 weeks after eosinophilia was noted |
| (22) | 59 | F | NR | SCC | NR | 10.3 | NR | NR | Eosinophil persisted until the patient’s death 2 months after diagnosis |
| (22) | 61 | M | NR | NR | NR | 14.4 | NR | NR | Patient died soon after presentation |
| (22) | 71 | M | NR | NR | NR | 1.7 | NR | NR | Patient deteriorated rapidly and died |
| (22) | 71 | M | NR | NR | IV | 5.9 | NR | NR | Patient died 2 weeks after diagnosis, when AEC had increased approximately 10-fold |
| (22) | 57 | M | NR | NR | NR | 8 | NR | NR | The patient deteriorated rapidly and died 3 months later, during which time moderate to severe eosinophilia persisted |
ADC, adenocarcinoma; AEC, absolute eosinophil count; BM, bone marrow; EOS, eosinophil; LCC, large-cell carcinoma; Met, metastasis; NR, not reported; SCC, squamous cell carcinoma.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-1089).
Case presentation
A 64-year-old man was admitted in Jun 2020 with shortness of breath. His ECOG performance status (PS) was evaluated at 1. Past medical history was significant for tobacco use of 100 pack years before quitting 10 years earlier and grade 3 hypertension under regular therapeutic control for more than ten years. Physical examination revealed no palpable supraclavicular lymph nodes, symmetric rib cage, dull note to percussion and absent breath sound over the left lung field, and resonate note and clear breath sound over the right lung field, while no rales or rhonchi were noted. There were leukocytosis (leukocyte count 13.61×109/L) and mild blood eosinophilia (AEC 1.38×109/L). Enhanced CT showed pleural effusion and metastases to multiple mediastinal lymph nodes, left pleura, diaphragm, and spleen (Figure 1A). Histopathological review of CT-guided lung biopsy indicated poorly differentiated adenocarcinoma consistent with stage IVB (T4N2M1c) and slightly infiltrated by eosinophils (Figure 1B). Immunostaining against PD-L1 showed a Tumor Proportion Score of 80%. In terms of cause of eosinophilia, the patient had no history of allergy or autoimmune conditions. Total IgE was normal and tests for major autoantibodies, including anti-neutrophil cytoplasm antibody (ANCA), anti-glomerular basement membrane (GBM), and antinuclear antibodies (ANAs), were all negative. The patient also tested negative for antibodies against hepatitis A/B/C virus, syphilis, and HIV, and tuberculosis infection was excluded via interferon gamma release assay. Particularly, Rose Bengal Plate and Brucella agglutination tests were performed considering the patient’s occupation as a sheep breeder, and both were negative, further excluding microbic infection. Stool examination for ova and parasites was also negative. Bone marrow aspiration on Jul 6 revealed a blast percentage of 0.5% and excluded leukemic process. The blood eosinophilia was therefore determined as tumor-associated.
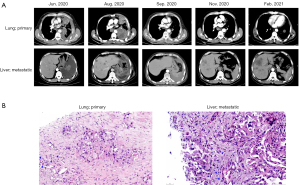
Genomic profiling with plasma using a panel of 168 cancer-associated genes (Burning Rock Biotech, China) identified EGFR p.L858R (abundance of 34.6%) and MET amplification (copy number 4.4). Meanwhile, eosinophilia continued to exacerbate with AEC peaking at 6.41×109/L (Figure 2). The patient was started on icotinib monotherapy (125 mg p.o, tid) since Jul. Follow-up CT scan in Aug showed enlarged pulmonary lesion and multiple metastases in the liver, peritoneum, and retroperitoneum, leading to an evaluation of progressive disease (PD; Figure 1A). Pathological examination of biopsy of a hepatic lesion confirmed the pulmonary origin and revealed eosinophil infiltration (Figure 1B). At this point, there was severe eosinophilia in peripheral blood with AEC peaking at 23.36×109/L (Figure 2). MET amplification is a major mechanism of acquired resistance to EGFR TKIs (27,28). For EGFR TKI-resistant lung adenocarcinoma with acquired MET amplification, a recent retrospective study of 11 patients showed an overall response rate of over 80% and median progression-free survival of 5.8 months achieved with first- or third-generation EGFR TKI plus ALK/ MET/ROS1 inhibitor crizotinib (29). As plasma and a liver metastasis both tested negative for EGFR p.T790M, the patient started on icotinib (125 mg p.o. tid) combined with crizotinib (250 mg p.o. bid). He was evaluated at partial response (PR) one month later but progressed again after another two months in November (Figure 1A), during which AEC dropped temporarily dropped to borderline normal level one before the PR evaluation and rose again to mild eosinophilia shortly after the PD evaluation (Figure 2). Molecular testing showed EGFR L858R at markedly decreased abundance (6.5%) and similar MET copy number (copy number 5.0) compared with baseline, suggesting MET amplification as a potent oncogenic driver at this point. The patient refused chemotherapy. For alternative choices, there have been preclinical evidence and isolated report of EGFR-mutated NCSLC with elevated MET signaling responding to EGFR TKI combined with inhibitors of vascular endothelial growth factor (VEGF), a downstream effector of MET (30,31). Anlotinib, a multi-kinase inhibitor TKI targeting VEGFR2/3 and other kinases, is approved in China for third-line treatment of advanced NSCLC and therefore added to form the 3rd-line therapy (icotinib: 125 mg p.o. tid; crizotinib: 250 mg p.o. bid; and anlotinib: 8 mg p.o. qd, days 1–14, 21 days as a cycle), which began in Jan 2021. Despite initial self-reported asymptomatic high functional status, radiographically findings were consistent with PD a month later based on lesion enlargement (Figure 1A). Eosinophilia also worsened from mild to moderate (Figure 2). The patient refused further treatment and was lost to subsequent follow-ups.

All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Increased AEC is either a result of leukemic proliferation of precursors in the bone marrow or a reaction to pathological processes such as tumor activity, parasitic infections, or allergy. In this case, medical history, tests for autoantibodies, common pathogens, Brucella, ova and parasites were all negative. Combined with absence of leukemic process in the BM, the blood eosinophilia was determined to be cancer-associated.
Prognostic impact of paraneoplastic blood eosinophilia appears to be tumor type-dependent. For instance, higher preoperative blood AEC was significantly associated with lower risk of recurrent primary breast cancer (32). In NSCLC, however, a review of literature suggests eosinophilia as a potential poor prognostic factor (3,33). Of patients whose survival outcome was available (19/24), all but two died or started hospice within 1 year after initial diagnosis or onset of eosinophilia (Table 1). Moreover, there was considerable temporal proximity between progression/relapse and alleviation of eosinophilia and of the malignancy. For instance, our case is characterized by steep turns in the AEC curve between Aug 5 and Aug 28, which corresponded to progression on first-line EGFR inhibitor and rapid response to second-line combination of EGFR and MET inhibitors (Figure 2). Additionally, in three patients who underwent tumor excision, AEC normalized after surgery (4,5,8), and eosinophilia resolution and tumor size and pleural effusion reduction followed chemotherapy initiation in another case (6). Conversely, Kodama et al. described recurrent eosinophilia that heralded detection of a metastasis, which was followed by worsening symptoms (5), and in Slungaard et al. imaging studies revealed new masses after eosinophilia relapse, along with fever, hepatomegaly, and pulmonary emboli (4).
Nonetheless, it is noteworthy that clinical outcome did not appear on strongly correlated with severity of eosinophilia, as illustrated by the vast AEC difference in the above mentioned three surgically treated patients (AEC at diagnosis 1.1, 5, and 90×109/L). Also, it remains unclear whether and how eosinophilia precedes or follows cancer progression, which warrants investigation into the biological relevance of circulating eosinophils.
The surge in blood eosinophil count is likely a reflection of increased eosinophil production in the BM and traffic on route to the tumor. A chemotactic factor that can stimulate eosinophil generation in human BM culture in patient-derived tumor tissue and/or serum has been noted in a handful of studies (4-8,10,18,20,34). Identity of this factor is yet to be established, as granulocyte-macrophage colony-stimulating factor (GM-CSF; molecular weight 14.5 kD), interleukin-5 (IL-5; 40–50 kD), and a polypeptide of 30–40 kD (20) have been reported. In a patient with stage IIIA large cell carcinoma, elevated IL-5 and AEC both dropped after tumor removal (Table 1) (8). In contrast, Lammel et al. reported no abnormal increase in IL-5 level, whereas GM-CSF staining of tumor samples indicated intracellular expression along with that of GM-CSF receptor (18). It is likely that multiple cytokines regulate eosinophil maturation and recruitment in manners dependent on the tumor microenvironment context.
Contrary to the trend toward negative prognostic impact summarized above, a recent development suggests eosinophilia may predict response to immune checkpoint blockade (ICB) with PD-(L)1 inhibitors (23-26). A retrospective analysis revealed significantly improved survival outcomes in patients who presented with blood eosinophilia during immunotherapy than those who did not, thereby suggesting a promising therapeutic option (23). Eosinophilia was present before immunotherapy in 10 of the 33 patients in the former group. More research is therefore awaited to define patients with baseline eosinophilia who are likely to benefit from ICB, since sustained eosinophilia could lead to severe organ damage such as altered mental state (9) and endomyocardial disease (20,21), and eventually fatality. Indeed, patients with eosinophilia during treatment were at significantly greater risk of developing immune-related adverse effects compared to those without (23). Interestingly, the authors also noted higher prevalence of eosinophilia before immunotherapy (10/121, 8.3%) than reported in solid tumors (~1%). This higher prevalence could be attributed to paraneoplastic leukocytosis, which is not uncommon in NSCLC and has been associated with high granulocyte colony stimulating factor (G-CSF) levels and poor prognosis (18). A study of 37,973 Japanese healthy adults found male gender and current smoking associated with elevated leukocyte count among other factors, although ex-smoking was not. Most of the NCSLC patients with eosinophilia in Table 1 were either non- or ex-smokers, and in our case, leukocyte count was normal despite eosinophilia at the three consecutive blood tests between start and progression on 2nd-line icotinib plus crizotinib (data not shown). More research is therefore needed to define clinicopathologic factors contributing to blood eosinophilia in NSCLC.
In summary, this case is, to the best of our knowledge, the first to provide evidence for blood eosinophilia paralleling cancer progression in an EGFR- and MET-altered NSCLC patient who received targeted therapy, which suggested possible negative prognostic impact of blood eosinophilia in driver-positive NSCLC.
Acknowledgments
We would like to thank the patient and his family.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-1089
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-1089). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jameson JL, Longo DL. Paraneoplastic syndromes: endocrinologic/hematologic. In: Fauci AS, Braunwald E, Kasper DL, et al. editors. Harrison's Principles of Internal Medicine. 17 ed. New York, NY: McGraw Hill Medical, 2008:617-22.
- Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res 2014;2:1-8. [Crossref] [PubMed]
- Sakkal S, Miller S, Apostolopoulos V, et al. Eosinophils in Cancer: Favourable or Unfavourable? Curr Med Chem 2016;23:650-66. [Crossref] [PubMed]
- Slungaard A, Ascensao J, Zanjani E, et al. Pulmonary carcinoma with eosinophilia. Demonstration of a tumor-derived eosinophilopoietic factor. N Engl J Med 1983;309:778-81. [Crossref] [PubMed]
- Kodama T, Takada K, Kameya T, et al. Large cell carcinoma of the lung associated with marked eosinophilia. A case report. Cancer 1984;54:2313-7. [Crossref] [PubMed]
- Matsumoto S, Tamai T, Yanagisawa K, et al. Lung cancer with eosinophilia in the peripheral blood and the pleural fluid. Intern Med 1992;31:525-9. [Crossref] [PubMed]
- Sawyers CL, Golde DW, Quan S, et al. Production of granulocyte-macrophage colony-stimulating factor in two patients with lung cancer, leukocytosis, and eosinophilia. Cancer 1992;69:1342-6. [Crossref] [PubMed]
- Pandit R, Scholnik A, Wulfekuhler L, et al. Non-small-cell lung cancer associated with excessive eosinophilia and secretion of interleukin-5 as a paraneoplastic syndrome. Am J Hematol 2007;82:234-7. [Crossref] [PubMed]
- El-Osta H, El-Haddad P, Nabbout N. Lung carcinoma associated with excessive eosinophilia. J Clin Oncol 2008;26:3456-7. [Crossref] [PubMed]
- Lo CH, Jen YM, Tsai WC, et al. Rapidly evolving asymptomatic eosinophilia in a patient with lung adenocarcinoma causes cognitive disturbance and respiratory insufficiency: Case report. Oncol Lett 2013;5:495-8. [Crossref] [PubMed]
- Nqwata L, Wong ML, Mohanlal R, et al. Hypereosinophilia as a paraneoplastic phenomenon in non-small cell lung carcinoma. S Afr Respir J 2015;21:108-9. [Crossref]
- Venkatesan R, Salam A, Alawin I, et al. Non-small cell lung cancer and elevated eosinophil count: A case report and literature review. Cancer Treat Commun 2015;4:55-8. [Crossref]
- Motilal B, Savita A, Takhar R. Peripheral eosinophilia in a case of adenocarcinoma lung: A rare association. The Journal of Association of Chest Physicians 2015;3:60-2. [Crossref]
- Abughanimeh O, Tahboub M, Abu Ghanimeh M. Metastatic Lung Adenocarcinoma Presenting with Hypereosinophilia. Cureus 2018;10:e2866. [PubMed]
- Livne Margolin M, Zeitlin N, Friedman YE, et al. Eosinophilia and Leukocytosis in a Patient with Lung Cancer. Isr Med Assoc J 2019;21:58-9. [PubMed]
- Akkad N, Jiang Y, Shin D. Leucocytosis and Stroke in a Lung Cancer Patient. Eur J Case Rep Intern Med 2020;7:001872. [PubMed]
- Verstraeten AS, De Weerdt A, van Den Eynden G, et al. Excessive eosinophilia as paraneoplastic syndrome in a patient with non-small-cell lung carcinoma: a case report and review of the literature. Acta Clin Belg 2011;66:293-7. [PubMed]
- Lammel V, Stoeckle C, Padberg B, et al. Hypereosinophilia driven by GM-CSF in large-cell carcinoma of the lung. Lung Cancer 2012;76:493-5. [Crossref] [PubMed]
- Saliba WR, Dharan M, Bisharat N, et al. Eosinophilic pancreatic infiltration as a manifestation of lung carcinoma. Am J Med Sci 2006;331:274-6. [Crossref] [PubMed]
- Jaski BE, Goetzl EJ, Said JW, et al. Endomyocardial disease and eosinophilia. Report of a case. Circulation 1978;57:824-7. [Crossref] [PubMed]
- Spry CJ, Weetman AP, Olsson I, et al. The pathogenesis of eosinophilic endomyocardial disease in patients with carcinomas of the lung. Heart Vessels 1985;1:162-9. [Crossref] [PubMed]
- Knox AJ, Johnson CE, Page RL. Eosinophilia associated with thoracic malignancy. Br J Dis Chest 1986;80:92-5. [Crossref] [PubMed]
- Alves A, Dias M, Campainha S, et al. Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy. J Thorac Dis 2021;13:2716-27. [Crossref] [PubMed]
- Krishnan T, Tomita Y, Roberts-Thomson R. A retrospective analysis of eosinophilia as a predictive marker of response and toxicity to cancer immunotherapy. Future Sci OA 2020;6:FSO608. [Crossref] [PubMed]
- Lou Y, Marin-Acevedo JA, Vishnu P, et al. Hypereosinophilia in a patient with metastatic non-small-cell lung cancer treated with antiprogrammed cell death 1 (anti-PD-1) therapy. Immunotherapy 2019;11:577-84. [Crossref] [PubMed]
- Baroz AR, Mambetsariev I, Fricke J, et al. Elevated Eosinophil Count Following Pembrolizumab Treatment for Non-Small Cell Lung Cancer. Cureus 2021;13:e16266. [Crossref] [PubMed]
- Zhong J, Li L, Wang Z, et al. Potential Resistance Mechanisms Revealed by Targeted Sequencing from Lung Adenocarcinoma Patients with Primary Resistance to Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs). J Thorac Oncol 2017;12:1766-78. [Crossref] [PubMed]
- Hammerman PS, Jänne PA, Johnson BE. Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. Clin Cancer Res 2009;15:7502-9. [Crossref] [PubMed]
- Wang Y, Tian P, Xia L, et al. The clinical efficacy of combinatorial therapy of EGFR-TKI and crizotinib in overcoming MET amplification-mediated resistance from prior EGFR-TKI therapy. Lung Cancer 2020;146:165-73. [Crossref] [PubMed]
- Seki N, Natsume M, Ochiai R, et al. Promising Combination Therapy with Bevacizumab and Erlotinib in an EGFR-Mutated NSCLC Patient with MET Amplification Who Showed Intrinsic Resistance to Initial EGFR-TKI Therapy. Case Rep Oncol 2019;12:91-7. [Crossref] [PubMed]
- Nakade J, Takeuchi S, Nakagawa T, et al. Triple inhibition of EGFR, Met, and VEGF suppresses regrowth of HGF-triggered, erlotinib-resistant lung cancer harboring an EGFR mutation. J Thorac Oncol 2014;9:775-83. [Crossref] [PubMed]
- Ownby HE, Roi LD, Isenberg RR, et al. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983;52:126-30. [Crossref] [PubMed]
- Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol 1981;34:1343-8. [Crossref] [PubMed]
- Watanabe M, Ono K, Ozeki Y, et al. Production of granulocyte-macrophage colony-stimulating factor in a patient with metastatic chest wall large cell carcinoma. Jpn J Clin Oncol 1998;28:559-62. [Crossref] [PubMed]


