
The detection rate of methylene blue combined with another tracer in sentinel lymph node biopsy of early-stage breast cancer: a systematic review and network meta-analysis
Introduction
Breast cancer is the most common malignant tumor occurring in women worldwide. In China, the incidence of breast cancer increases every year (1). The eighth edition of the American Joint Committee on Cancer (AJCC)’s Cancer Staging Manual provides comprehensive advice on the staging, prognosis, and treatment of cancer, and is considered more accurate than traditional anatomical staging (2). Axillary lymph node (ALN) status is an important factor in breast cancer staging and prognosis; therefore, the accurate evaluation of ALN status is essential for the formulation an appropriate treatment plan. Sentinel lymph node biopsy (SLNB) has become the standard staging scheme for patients with cN0 early-stage breast cancer (3,4). Improving the identification rate (IR), sensitivity (SEN), and accuracy rate (AR) of SLNB while simultaneously reducing the false-negative rate (FNR) is a critical concern for surgeons, and the selection of tracers is key to the success of SLNB.
Common tracers for SLNB include blue dye, radioisotope, and fluorescence, or blue dye combined with radioisotope or fluorescence. The combination of radioisotope and blue dyes, such as patent blue or isosulfan blue, is considered the standard mapping method worldwide. Meanwhile, the combined use of indocyanine green (ICG) and blue dye has gradually become more frequent in clinical practice as a means of improving detection rates (5). However, hospitals in many developing countries, including China, have limited access to patent blue or isosulfan blue and are unable to provide the personnel and equipment required for radioisotope use. Consequently, with regard to the selection of blue dye, the 2021 Chinese Society of Breast Surgery (CSBrS) practice guidelines recommend blue dye alone or fluorescence alone as class IA, radioisotope alone or the combination of radioisotope and blue dye as class IB (6).
Researchers have sought to improve the detection rate of SLNB by combining different tracers. In previous studies, methylene blue (MB) combined with 99mtechnetium-labeled sulphur colloid (MB + Tc99m) and MB combined with ICG (MB + ICG) showed certain advantages over MB alone. However, as these studies were small in scale and technically heterogeneous, they did not provide clear results. The detection rates of MB alone, MB + Tc99m, and MB + ICG are therefore uncertain. We thus sought to evaluate the detection rates of these 3 methods and to examine their differences using a network meta-analysis (NMA).
We present the following article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-1239), and our protocol was registered with the International Platform of Registered Systematic Review and Meta-Analysis Protocols (INPLASY; registration no. INPLASY202150107).
Methods
Literature retrieval strategy
This meta-analysis was reported and screened according to the PRISMA Guidelines (7). We conducted a comprehensive electronic literature search on the PubMed, Embase, Web of Science, CNKI, and Wanfang Data databases from inception to October 2021. The following search terms from the Medical Subject Headings (MeSH) vocabulary were used: “Breast Neoplasms”, “Methylene Blue”, and “Sentinel Lymph Node Biopsy”. The Chinese databases were searched with the equivalent Chinese keywords to those from the English databases. In addition, the reference lists of previous reviews were also reviewed for plausible articles. Letters, editorials, case reports, and reviews were excluded from the study. We did not attempt to obtain any unpublished research. Any disagreements were resolved through discussion.
Eligibility criteria
Inclusion criteria
The inclusion criteria for literature were the following: all patients examined were diagnosed with early-stage breast cancer by cytology or histopathology; at least 1 group in the study underwent MB alone, MB + ICG, or MB + Tc99m as a mapping method for SLNB; some or all of the IR, SEN, AR, and FNR indicators could be extracted or calculated from the study; the study was a cohort study or case–control study; and the study publication language was English or Chinese.
Exclusion criteria
Studies that included clinical node-positive patients (cN+), distant metastasis, or surgical contraindications for SLNB were excluded. Patients who received neoadjuvant chemotherapy or radiotherapy before SLNB were also excluded. Studies that used other blue dyes, such as patent blue or isosulfan blue, were excluded. In terms of outcomes, studies that lacked available data were excluded. Studies that consisted of letters, editorials, case reports, or reviews were excluded. For studies with overlapping patients or repeated reports, only studies with the largest number of patients were included.
The retrieval strategy is shown in Figure 1.
Selection process and data collection process
The data were extracted by 2 independent reviewers (HJL and MSS) and verified for accuracy by 2 other reviewers. Any disagreements were resolved through discussion. Summaries of study characteristics included the first author, publication year, study origin, the age of the patients, the tumor stage, the mapping method for SLNB, and the number of patients enrolled.
Risk of bias assessment
The risk of bias in the studies was assessed with Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2), a standardized tool for evaluating the quality of diagnostic accuracy studies (8). QUADAS-2 contains 4 domains for assessing the risk of bias: patient selection, index test, reference standard, and flow and timing. Signaling questions (yes/no/unclear) are used to assess the risk of bias in each domain. If the answers to all signaling questions in a domain are yes, then the risk of bias can be judged as low. If any signaling question is answered no, then the potential for bias exists. Review authors must then use the guidelines developed in phase 2 to judge the risk of bias. The “unclear” answer is used when insufficient data are reported to allow a judgment. The first 3 domains, patient selection, index test, and reference standard, are further assessed in terms of the applicability of the study to the research question. All studies in this meta-analysis were independently analyzed by 2 independent reviewers (HJL and MSS). The questions adopted in our review are listed in Table S1 and the outcome in our review are listed in Table S2.
Statistical analysis
In this study, IR was defined as the number of patients for whom SLNs were successfully identified divided by the total number of patients who underwent SLNB. AR was defined as the proportion of people whose ALN status were correctly predicted by SLNB. The results of each successfully identified SLN were further classified as true positive (TP), true negative (TN), or false negative (FN). We then evaluated 2 diagnostic parameters: FNR [FN/(FN + TP)] and sensitivity [TP/(TP + FN)].
The R meta4diag package version 3.6.3 (https://www.r-project.org) was used to perform the pooled analyses of FNR and SEN, which were considered to be diagnostic parameters in this study. The pooled analyses of IR and AR, which were single proportions, were conducted using the “metaprop” function in the R meta package. The logit transformation was implemented to calculate overall proportions. The method of inverse variance was conducted for the pooling of individual studies. The inconsistency statistic (I2) was used to evaluate the heterogeneity among the studies. The random-effects model was adopted if I2 was >50%; otherwise, the fixed effects model was used. Potential publication bias was determined by a funnel plots and assessed using Begg’s test.
Mixed-comparison analysis using random-effects models, i.e., the NMA was conducted for comparison of IR and AR across the tracers. The NMA was carried out with a random-effects model of the Bayesian framework analysis using the “GeMTC” R package, which includes the software JAGS 4.3.0. Odds ratios (OR) and their 95% CI were applied for the comparisons of IR and AR between the 3 mapping methods.
In this study, all statistical tests were 2 sided, and P values of less than 0.05 were deemed significant.
Results
Basic characteristics of included studies
Our meta-analysis included 7,498 patients in 49 studies published between inception and 2021, of which 43 studies were from China, 4 from India, and 1 each from Turkey and Italy. At least 1 group of patients in 26 studies were subjected to MB + Tc99m in SLNB, and at least 1 group of patients in 35 studies were subjected to MB + ICG in SLNB. Table 1 lists the basic characteristics of 49 studies, while the retrieval strategy is shown in Figure 1.
Table 1
| No. | Study | Year | Origin | Age [year, range] | Tumor stage | The mapping method | No. of patients |
|---|---|---|---|---|---|---|---|
| 1 | Tang et al. (9) | 2005 | China | 45a [29–65] | T1-2 | MB + Tc99m | 83 |
| MB | 38 | ||||||
| 2 | Zhao et al. (10) | 2005 | China | NR | T1-3 | MB + Tc99m | 38 |
| 3 | Lu et al. (11) | 2006 | China | 48.7 [32–73] | T1-2 | MB + Tc99m | 120 |
| 4 | D’Eredita et al. (12) | 2006 | Italy | 57 [27–87] | T1-2 | MB + Tc99m | 40 |
| 57.6 [40–78] | MB | 40 | |||||
| 5 | Liu et al. (13) | 2007 | China | 50±10 | T1-2 | MB + Tc99m | 60 |
| 52±12 | MB | 104 | |||||
| 6 | Lin et al. (14) | 2007 | China | 44±15.8 [33–74] | T1-2 | MB + Tc99m | 112 |
| 7 | Somashekhar et al. (15) | 2008 | India | 52 [24–82] | T1-2 | MB + Tc99m | 100 |
| 8 | Wang et al. (16) | 2009 | China | 52 [34–78] | T1-2 | MB + Tc99m | 37 |
| MB | 34 | ||||||
| 9 | Chen et al. (17) | 2009 | China | 46 [32–58] | T1-2 | MB + Tc99m | 13 |
| MB | 7 | ||||||
| 10 | Yang et al. (18) | 2010 | China | 45 [24–73] | T1-2 | MB + Tc99m | 109 |
| 11 | Liu et al. (19) | 2010 | China | 52.7 [36–75] | T1-2 | MB + Tc99m | 36 |
| 12 | Chen et al. (20) | 2011 | China | 31–72 | T1-2 | MB + Tc99m | 31 |
| 13 | Coskun et al. (21) | 2012 | Turkey | 49.8 [27–74] | T(NR) | MB + Tc99m | 47 |
| MB | 53 | ||||||
| 14 | Lu et al. (22) | 2012 | China | 45a [26–76] | T1-2 | MB + Tc99m | 65 |
| 15 | Tian et al. (23) | 2012 | China | 48a [19–85] | T1-2 | MB + Tc99m | 199 |
| MB | 199 | ||||||
| 16 | Cao et al. (24) | 2014 | China | 52a [29–81] | T1-2 | MB + ICG | 107 |
| MB | 107 | ||||||
| 17 | Zhang et al. (25) | 2015 | China | 45.6±8.5 [27–68] | T1-2 | MB + Tc99m | 40 |
| 18 | Ji et al. (26) | 2015 | China | 53.00±11.2 [28–71] | T1-3 | MB + ICG | 65 |
| 19 | Lei et al. (27) | 2015 | China | 22–80 | T1-2 | MB + Tc99m | 195 |
| 20 | Yuan et al. (28) | 2016 | China | 48 [22–77] | T1-2 | MB + Tc99m | 52 |
| MB + ICG | 52 | ||||||
| 21 | Zhang et al. (29) | 2016 | China | NR | T1-2 | MB + ICG | 131 |
| MB | 145 | ||||||
| 22 | Liu et al. (30) | 2016 | China | 50.21±8.73 | T1-2 | MB + ICG | 62 |
| 49.73±9.60 | MB | 62 | |||||
| 23 | Cui et al. (31) | 2016 | China | 49.58±6.39 [28–71] | T1-2 | MB + ICG | 100 |
| 50.11±6.80 [26–75] | T1-2 | MB | 100 | ||||
| 24 | Tang et al. (32) | 2016 | China | 45.6±12.9 | T(NR) | MB + ICG | 95 |
| 46.2+15.9 | T(NR) | MB | 65 | ||||
| 25 | Zhang et al. (33) | 2016 | China | NR | T1-2 | MB + ICG | 131 |
| MB | 145 | ||||||
| 26 | Guo et al. (5) | 2017 | China | 52 [33–74] | T1-2 | MB + ICG | 198 |
| MB | 198 | ||||||
| 27 | Ji et al. (34) | 2017 | China | 53±11.2 | T1-3 | MB + ICG | 65 |
| 28 | Heng et al. (35) | 2017 | China | NR | T1-2 | MB + ICG | 46 |
| MB | 74 | ||||||
| 29 | Sun et al. (36) | 2017 | China | NR | T(NR) | MB + ICG | 85 |
| MB | 85 | ||||||
| 30 | Yuan et al. (37) | 2019 | China | 52.6±10.8 | T1-3 | MB + ICG | 245 |
| MB | 38 | ||||||
| 31 | Agarwal et al. (38) | 2018 | India | NR | T(NR) | MB + Tc99m | 78 |
| 32 | Shen et al. (39) | 2018 | China | 47.8±10.8 | T1-2 | MB + ICG | 374 |
| 47.2±9.7 | MB | 149 | |||||
| 33 | Li et al. (40) | 2018 | China | 54.3+1.6 | T1-2 | MB + ICG | 85 |
| 54.1±1.8 | MB | 85 | |||||
| 34 | Zhang et al. (41) | 2018 | China | 47.52±5.78 | T1-3 | MB + ICG | 136 |
| 48.52±6.30 | MB | 132 | |||||
| 35 | Lei et al. (42) | 2015 | China | 63.7 [61–69] | T1-2 | MB + ICG | 63 |
| 36 | Gupta et al. (43) | 2020 | India | 54.5 [53.5±11.05] | T1-2 | MB + Tc99m | 30 |
| 53.5 [56.6±11.26] | MB | 30 | |||||
| 37 | Qin et al. (44) | 2019 | China | NR | T1-3 | MB + ICG | 60 |
| MB | 60 | ||||||
| 38 | Zhou et al. (45) | 2019 | China | 46.9±15 | T1-3 | MB + ICG | 316 |
| 39 | Zhu et al. (46) | 2019 | China | 46.3 | T1-2 | MB + ICG | 105 |
| 48.3 | MB | 101 | |||||
| 40 | Zhu et al. (47) | 2019 | China | 46.2±15.9 [33–74] | T(NR) | MB + ICG | 95 |
| 45.6±12.9 [32–75] | MB | 65 | |||||
| 41 | Zhao et al. (48) | 2019 | China | 47.53±5.45 | T(NR) | MB + ICG | 86 |
| 48.02±5.27 | MB | 86 | |||||
| 42 | Liu et al. (49) | 2019 | China | 52.5±17.5 | T1-2 | MB + ICG | 70 |
| 52.3±17.4 | MB | 70 | |||||
| 43 | Zhou et al. (50) | 2019 | China | 52.8 [27–78] | T(NR) | MB + ICG | 140 |
| MB | 140 | ||||||
| 44 | Gong et al. (51) | 2019 | China | NR | T1-2 | MB + Tc99m | 43 |
| 45 | Huang et al. (52) | 2019 | China | 20–70 | T1-2 | MB + ICG | 20 |
| 46 | Bai et al. (53) | 2020 | China | 52.4±9.8 | T1-2 | MB + ICG | 57 |
| 53.1±8.4 | MB | 57 | |||||
| 47 | Huang et al. (54) | 2020 | China | 51.8±5.2 | T1-2 | MB + ICG | 50 |
| 50.6±4.9 | MB | 50 | |||||
| 48 | Zhang et al. (55) | 2021 | China | 30–77 [M46.5] | T1-2 | MB + ICG | 197 |
| MB | 218 | ||||||
| 49 | Fang et al. (56) | 2021 | China | NR | T1-2 | MB + Tc99m | 92 |
| MB | 92 |
a, Median. NR, no record; MB, methylene blue; ICG, indocyanine green; Tc99m, 99m Technetium-labeled Sulphur Colloid.
Identification Rate
IR with MB + Tc99m
Twenty-two studies reported the IR, and with low heterogeneity (I2=19%, Pheterogeneity=0.21). A fixed-effects model was used to estimate the IR with MB + Tc99m, with a result of 96% (95% CI: 95–97%; Figure 2A).
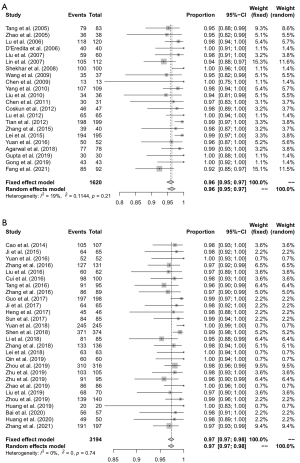
IR with MB + ICG
Twenty-eight studies reported the IR, and with low heterogeneity (I2=0%, Pheterogeneity=0.74). A fixed-effects model was used to estimate the IR with MB + ICG, with a result of 97% (95% CI: 97–98%; Figure 2B).
False-negative rate
Seventeen studies reported FNR that ranged from 0% to 14%. The summary estimates of FNR with MB + Tc99m were 7% (95% CI: 5–10%; Figure 3A). Eight studies reported FNR that ranged from 3% to 11%. The summary estimates of FNR with MB + ICG were 7% (95% CI: 4–10%; Figure 3B).

Sensitivity
Seventeen studies reported SEN that ranged from 86% to 96%. The summary estimates of SEN with MB + Tc99m were 93% (95% CI: 90–95%; Figure 4A). Eight studies reported SEN that ranged from 89% to 97%. The summary estimates of SEN with MB + ICG were 93% (95% CI: 90–96%; Figure 4B).

Accuracy
Using the random-effects model to estimate the AR with MB + Tc99m produced a result of 96% (95% CI: 94–97%, I2=0%; Pheterogeneity =0.86; Figure 5A). Using the fixed-effects model to estimate the AR with MB + ICG produced a result of 97% (95% CI: 96–98%; I2=0%, Pheterogeneity =0.88; Figure 5B).

Network meta-analysis
We wanted to simultaneously assess and compare the detection rate among the tracer methods of MB, MB + Tc99m, and MB + ICG. However, studies directly comparing MB + Tc99m and MB + ICG are scarce. We found that 31 of the 49 studies included at least 2 groups of patients who used MB alone and MB + Tc99m or MB + ICG. We therefore conducted an NMA, in pairwise comparison: if MB participated in the comparison, then MB was taken as the reference; otherwise, MB + Tc99m was taken as the reference.
Mixed-comparison analysis using random-effects models was conducted for comparison of IR and AR across three tracers. Compared with MB alone, MB + Tc99m (OR, 4.66; 95% CI: 2.19–10.08) and MB + ICG (OR, 6.17; 95% CI: 4.02–10.29) contributed to higher IR. No statistical significance was found in comparison between MB + Tc99m and MB + ICG (OR, 1.33; 95% CI: 0.56–3.32). With regard to AR, significant difference was only observed between MB and MB + ICG (OR, 2.89; 95% CI: 1.51–5.75), indicating a higher AR when using MB + ICG as the tracer. No significant difference was found in comparison between MB and MB + Tc99m (OR, 2.12; 95% CI: 0.84–5.81), or between MB + Tc99m and MB + ICG (OR, 1.37; 95% CI: 0.41–4.20).
Table 2 gives the estimated mean difference in accuracy rate (top right) and identification rate (bottom left) between each combination of mapping methods obtained from mixed-comparison models.
Table 2
| Mapping method | MB | MB + Tc99m | MB + ICG |
|---|---|---|---|
| MB | – | 2.12 (0.84–5.81) | 2.89 (1.51–5.75) |
| MB + Tc99m | 4.66 (2.19–10.08) | – | 1.37 (0.41–4.20) |
| MB + ICG | 6.17 (4.02–10.29) | 1.33 (0.56–3.32) | – |
Above the leading diagonal are the estimates of the mean difference in accuracy rate (95% CI), and below the leading diagonal are the estimates of the mean difference in identification rate. Results are presented as odds ratios and 95% confidence intervals. MB, methylene blue; Tc99m, 99m technetium-labeled sulphur colloid; ICG, indocyanine green.
Quality assessment of included studies and publication bias
QUADAS-2 was used to assess the quality of each study, and these results are listed in Table S2. All the studies had a high risk of patient selection bias, as they had a case–control design. Some studies had a high risk or an unclear risk of flow and timing bias, mainly due to the advancement of surgical treatment methods for breast cancer and not all patients having received axillary lymph node dissection (ALND). All other risks were rated as low.
Since the number of articles that each research indicator was reported were varied, we used IR, which was reported in the highest number of studies, to evaluate publication bias. The left and right sides in the IR funnel plot are nearly symmetrical, which suggests that there was a low possibility of publication bias (Figure 6A,6B). The Begg’s test values of IR using MB + Tc99m and MB + ICG were P=0.17 and P=0.04, respectively, which suggests that there also was a low possibility of publication bias of MB + ICG.

Discussion
The National Comprehensive Cancer Network (NCCN) and other guidelines agree that SLNB should be the standard method used for ALN staging in cN0 early-stage breast cancer, and that patients who are SLNB negative can be exempted from ALND (4,6,57-60). As a common tracer for SLNB, blue dye has been used widely in clinical practice, either alone or in combination with other tracers. The standard blue dyes, patent blue or isosulphan blue, combined with radioisotope tracers is the preferred trace method recommended by the American Society of Clinical Oncology (ASCO) for SLNB (61). However, for reasons involving the availability of drugs and health economics, these two standard tracers cannot be used clinically in many developing countries, including China.
Studies have demonstrated that the IR and FNR of MB used as a substitute for blue dye in SLNB show no clinical or statistical differences when compared with isosulphan blue (62). A meta-analysis of 18 studies from 2000 to 2017 found that when MB alone was used, the IR was 91% and the FNR was 13%, with a FNR of <10% reported in the past 5 years. These rates conform to the recommended standards of the American Society of Breast Surgeons (ASBrS) (63). MB alone is therefore a safe and effective alternative to standard blue dyes in the clinical practice of SLNB.
To further improve IR and reduce FNR, MB has been combined with other tracers, including radioisotopes and fluorescent tracers, which are now used widely in clinical practice. Previous systematic reviews and meta-analyses have investigated the use of MB, ICG, and Tc99m in SLNB. Wang et al. (64) conducted a meta-analysis of 15 studies from China. The results showed that the detection rate, number of detections, sensitivity, and specificity of MB + ICG were significantly increased compared with MB alone, while the FNR decreased significantly. A systematic review published by Kim et al. (65), which included 69 studies investigating SLNB and ALND of early-stage breast cancer, concluded that the dual-tracer mapping method had a higher IR compared with radioisotope or blue dye alone.
The present study evaluated studies that included both MB and MB + Tc99m or MB + ICG. Our NMA showed that the IR, FNR, SEN, and AR using MB + Tc99m were 96%, 7%, 93%, and 96%, respectively, while the IR, FNR, SEN, and AR using MB + ICG were 97%, 7%, 93%, and 97%, respectively. These results are superior to the IR and FNR of the MB single-tracer mapping method reported in previous literature (63).
IR and FNR are important indicators for evaluating the effectiveness of the tracer in SLNB. It is important to note that many factors can influence IR; for example, research has shown that experienced surgeons can achieve a 95.6% IR with blue dye alone (66). However, this meta-analysis suggests that MB combined with Tc99m or ICG can achieve a higher overall IR, which is consistent with the conclusions of studies using other blue dyes combined with radioisotopes or fluorescent tracers (67-69).
With respect to FNRs, the ASBrS previously stated that an FNR below 5% could only be accepted when the AR was greater than 95% (70). However, most recent studies have reported an FNR between 5% and 10%. Our research found that the FNR of both MB + Tc99m and MB + ICG was 7%. Therefore, in terms of clinical practice, the dual-tracer mapping method has significant advantages compared with the use of MB alone, which was shown to have a FNR of 13% in a previous meta-analysis. Wong et al. (71) found that when 1 SLN was obtained, the FNR was 14.3%, and that when 2 or more SLNs were obtained, the FNR was 4.3%. Among the 40 studies we included that reported the number of SLNs, only 6 studies reported fewer than 2 SLNs after using MB + Tc99m or MB + ICG. However, it should be noted that not all studies clearly indicated that the identification of SLN involved intraoperative pathological evaluation, and that the studies demonstrated differences in the pathological evaluation of positive lymph nodes. Therefore, we believe that the FNR of MB + Tc99m or MB + ICG is higher than 5% or 4.3%, as the studies in the meta-analysis included some retrospective studies of small samples which did not report the number of SLNs obtained, the pathological evaluation criteria, or whether or not intraoperative pathological evaluation occurred.
When comparing MB + Tc99m and MB + ICG, MB + ICG has some advantages over MB + Tc99m in IR and AR. Compared with the limitations radionuclide use in clinical practice, such as the high requirements of personnel qualification and management, the high price of equipment and tracers, the difficulty of storage, and the potential radioactive damage to patients and staff, the use of ICG is easier to promote. The findings of this study provide evidence-based support for the clinical application of MB + ICG.
Although no significant heterogeneity was found in this study, the AR (I2=61%, P<0.01) using MB + Tc99m demonstrated a degree of heterogeneity. Despite the utility of sensitivity analysis and meta regression, the origin of the heterogeneity could not be thoroughly traced. Many of the studies included in this meta-analysis were retrospective studies of relatively low quality. In terms of publication bias, our application of Begg’s tests using the IR of MB + Tc99m (P=0.17) or MB + ICG (P=0.04) show less possibility of publication bias; meanwhile, potential bias may exist due to the tendency for positive results to be published and our strict inclusion criteria. This study also limited the publication language to English or Chinese, so publication bias cannot be totally excluded.
This evidence-based study has demonstrated that the MB single-tracer method can be used safely in clinical practice, especially in areas where access to other tracers is limited (72). Our meta-analysis showed that the MB + Tc99m or MB + ICG mapping methods can be used to obtain higher IR and lower FNR than MB alone. Our NMA showed no statistical significance between MB + Tc99m and MB + ICG with IR and AR.
Acknowledgments
We sincerely thank all the reviewers and editors for their supportive comments.
Funding: This work was funded by The Beijing Medical Award Foundation (Grant number: YXJL-2016-0040-0065).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-1239
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-1239). HJL, MSS, ZHY, XXC, QL, YJC, LX, YHL, and JMY report receiving funding from The Beijing Medical Award Foundation (Grant number YXJL-2016-0040-0065). LYL has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1-12. [Crossref] [PubMed]
- Ye J, Wang W, Xu L, et al. A retrospective prognostic evaluation analysis using the 8th edition of American Joint Committee on Cancer (AJCC) cancer staging system for luminal A breast cancer. Chin J Cancer Res 2017;29:351-60.
- Lyman GH, Somerfield MR, Giuliano AE. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: 2016 American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract 2017;13:196-8. [Crossref] [PubMed]
- Curigliano G, Burstein HJ, P, Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2019;30:1181. [Crossref] [PubMed]
- Guo J, Yang H, Wang S, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol 2017;15:196. [Crossref] [PubMed]
- Ye JM, Guo BL, Liu Q, et al. Clinical practice guidelines for sentinel lymph node biopsy in patients with early-stage breast cancer: Chinese Society of Breast Surgery (CSBrS) practice guidelines 2021. Chin Med J (Engl) 2021;134:886-94. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Tang J, Yang MT, Fan W, et al. Detection of sentinel lymph node in patients with early stage breast cancer. Ai Zheng 2005;24:1111-4. [PubMed]
- Zhao GR, Wang YD, Chen YD, et al. Methylene blue staining combined with nuclide using in the sentinel lymph node biopsy for patients with breast cancer. Lingnan Modern Clinics in Surgery 2005;120-1.
- Lu X, Li B, Hua B, et al. Clinical significance of sentinel lymph node biopsy in breast cancer. Chinese Journal of Practical Surgery 2006;26:860-1.
- D'Eredita G, Giardina C, Guerrieri AM, et al. A further validation of subareolar injection technique for breast sentinel lymph node biopsy. Ann Surg Oncol 2006;13:701-7. [Crossref] [PubMed]
- Liu ZB, Wu J, Huang XY, et al. Methylene blue versus combined blue dye-radioactive tracer in the detection of sentinel lymph node in breast cancer. Chinese Journal of General Surgery 2007;22:840-3.
- Lin LJ, Li SC, Xu ZY, et al. Application of nuclide and Methylene blue combined tracer in sentinel lymph node biopsy of breast cancer. Shandong Medical Journal 2007;73-4.
- Somashekhar SP, Zaveri Shabber S, Udupa Venkatesh K, et al. Sentinel lymphnode biopsy in early breast cancer using methylene blue dye and radioactive sulphur colloid - a single institution Indian experience. Indian J Surg 2008;70:111-9. [Crossref] [PubMed]
- Wang JF, Xiang FH, Ye Wang, et al. Influence of different tracers on identification rate and false negative rate of sentinel lymph node in breast cancer. Chinese Journal of Clinical Oncology and Rehabilitation 2009;16:511-3.
- Chen XW, He XQ, Liu XH. A clinical study of sentinel lymph node biopsy in breast cancer. The Journal of Practical Medicine 2009;25:2690-1.
- Yang YP, Zheng G, Zheng MZ, et al. Anatomic location and clinical significance of sentinel lymph node in primary breast cancer. Chinese Journal of Cancer Prevention and Treatment 2010;17:1100-3.
- Liu JK, Yu ZQ, Wu JC, et al. The role of sentinel lymph node biopsy on advising axi0llary lymph node dissection in breast cancer. Journal of Clinical and Experimental Medicine 2010;9:677-8.
- Chen X, Shi J, Zhou SJ, et al. Application of sentinel lymph node biopsy through sentinel lymph node pathway to guide axillary lymph node staging of breast cancer (Report of 30 cases). Journal of Nanjing Medical University 2011;31:440-2.
- Coskun G, Dogan L, Karaman N, et al. Value of sentinel lymph node biopsy in breast cancer patients with previous excisional biopsy. J Breast Cancer 2012;15:87-90. [Crossref] [PubMed]
- Lu ZD. Application of Radiocolloid Combined with Methylene Blue in Sentinel Lymph Node Biopsy for Patients with Early Breast Cancer. Journal of Chinese Oncology 2012;18:602-4.
- Tian CX, Chen J, Wei B, et al. Clinical Application of Combination of Radiolabeled Colloid and Blue Dye in Sentinel Lymph Node Biopsy for Early-Stage Breast Cancer. Chinese Journal of Bases and Clinics in General Surgery 2012;19:23-7.
- Cao YM, Wang S, Guo JJ, et al. Combination of ICG and methylene blue for mapping sentinel lymph nodes in early breast cancer patients. Chinese Journal of General Surgery 2014;29:119-22.
- Zhang YS, Liang QQ, Zhong L, et al. Evaluation of the tracing effect of carbon nanoparticles and methylene blue combined with 99 Tcm -sulfur colloid in endoscopic sentinel lymph node biopsy for breast cancer. Chinese Journal of Breast Disease(Electronic Edition) 2015;9:231-5.
- Ji YN, Jiang Y, Wei W, et al. Study of fluorescence navigation technology with indocyanine-green combined with mapping with methylene blue applied to sentinel lymph nodes biopsy in breast cancer patients. Guangxi Medical Journal 2015;37:1275-7.
- Lei SG, Yu XF, Xie CW, et al. Application of radionuclide imaging and methylene blue staining in the detection of sentinel lymph nodes in breast cancer. Jiangxi Medical Journal 2015;50:31-3.
- Yuan L, Zhou Y, Hu Y, et al. Indocyanine green combined with methylene blue for sentinel lymph node biopsy in breast cancer patients. Chinese Journal of Breast Disease(Electronic Edition) 2016;10:87-91.
- Zhang JZN, Ou JH, Zhang CG, et al. Combined tracing method of indocyanine green fluorescence and methylene blueing in sentinel lymph node biopsy of breast cancer. Chinese Journal of General Surgery 2016;25:705-10.
- Liu JT, Guo WB, Sun JY. The value of indocyanine green combined with Methylene blue in SLNB detection in breast cancer. Chinese Journal of General Surgery 2016;25:1658-61.
- Cui RZ, Yang JH, Pan CX. Application of Indocyanine green joint methylene blue for sentinel lymph node biophy in patients with breast cancer. Journal of Hainan Medical College 2016;22:1584-6.
- Tang W, Zhang XL, Zeng FY. Application of indocyanine green in sentinel lymph node biopsy for breast cancer. Journal of Regional Anatomy and Operative Surgery 2016;25:395-7.
- Zhang ZC, Xie PZ, Chen JX, et al. Clinical value of combining indocyanine green fluorescence navigation with blue dye in sentinel lymph node biopsy in patients with breast cancer. Chinese Journal of Clinical Oncology 2016;43:757-60.
- Ji Y, Luo N, Jiang Y, et al. Clinical utility of the additional use of blue dye for indocyanine green for sentinel node biopsy in breast cancer. J Surg Res 2017;215:88-92. [Crossref] [PubMed]
- Heng RJ, Qi P, Feng YQ, et al. Comparison study of indocyanine green joint methylene blue with methylene blue alone for sentinel lymph node biopsy in patients with breast cancer. Henan Medical Research 2017;26:3073-5.
- Sun XL, Huang GL, Shen J, et al. The value of indocyanine green combined with methylene blue in sentinel lymph node biopsy in early breast cancer. Shandong Medical Journal 2017;57:48-50.
- Yuan Q, Wu G, Xiao SY, et al. Surgical Management of the Axilla in Breast Cancer Patients with Negative Sentinel Lymph Node: A Method to Reduce False-Negative Rate. World J Surg 2019;43:1047-53. [Crossref] [PubMed]
- Agarwal G, Rajan S, Mayilvaganan S, et al. Prospective Randomized Trial of Use of In-House Prepared Low-Cost Radiopharmaceutical Versus Commercial Radiopharmaceutical for Sentinel Lymph Node Biopsy in Patients with Early Stage Invasive Breast Cancer. World J Surg 2018;42:1391-5. [Crossref] [PubMed]
- Shen S, Xu Q, Zhou Y, et al. Comparison of sentinel lymph node biopsy guided by blue dye with or without indocyanine green in early breast cancer. J Surg Oncol 2018;117:1841-7. [Crossref] [PubMed]
- Li SQ, Su GS, Cheng SP, et al. Clinical value of indocyanine green combined with methylene blue in axillary sentinel lymph node biopsy of breast cancer. Shanxi Medical Journal 2018;47:2306-8.
- Zhang HD, Liu GQ, Zhang XF, et al. The application value of fluorescence imaging combined with methylene blue staining in sentinel lymph node biopsy of early breast cancer. Anhui Medical Journal 2018;39:1520-2.
- Lei SG, Yu XF, Xie CW, et al. Application of indocyanine green combined with methylene blue staining in sentinel lymph node biopsy of elderly breast cancer. Jiangxi Medical Journal 2015;53:1084-6.
- Gupta V, Raju K, Rao TS, et al. A Randomized Trial Comparing the Efficacy of Methylene Blue Dye Alone Versus Combination of Methylene Blue Dye and Radioactive Sulfur Colloid in Sentinel Lymph Node Biopsy for Early Stage Breast Cancer Patients. Indian J Surg Oncol 2020;11:216-22. [Crossref] [PubMed]
- Qin X, Yang M, Zheng X. Comparative study of indocyanine green combined with blue dye with methylene blue only and carbon nanoparticles only for sentinel lymph node biopsy in breast cancer. Ann Surg Treat Res 2019;97:1-6. [Crossref] [PubMed]
- Zhou Y, Li Y, Mao F, et al. Preliminary study of contrast-enhanced ultrasound in combination with blue dye vs. indocyanine green fluorescence, in combination with blue dye for sentinel lymph node biopsy in breast cancer. BMC Cancer 2019;19:939. [Crossref] [PubMed]
- Zhu XF, Zeng LL, Huang LL, et al. Indocyanine green in addition to methylene blue for sentinel lymph node biopsy in patients with early breast cancer. journal of Zunyi Medical University 2019;42:80-83.
- Zhu J. Application of indocyanine green combined with methylene blue in sentinel lymph node biopsy of breast cancer. Journal of Clinical Research 2019;036:113-5.
- Zhao XC. Value of indocyanine green and methylene blue in sentinel lymph node biopsy of breast cancer. Yiyao Qianyan 2019;009:101-2.
- Liu JT, Liu JQ, Guo WB. The significance of indocyanine green combined with Methylene blue in axillary sentinel lymph node biopsy of breast cancer. Guide of China Medicine 2019;17:54-5.
- Zhou R, Huang Y, Huang Y, et al. Application of Indocyanine Green combined with Methylene Blue in sentinel lymph node biopsy of breast cancer. Acta Medicinae Sinica 2019;32:96-100.
- Gong YH. Effect analysis of radionuclides combined with methylene blue in sentinel lymph node biopsy of breast cancer. Journal of Medical Aesthetice and Cosmetology 2019;28:49.
- Huang XH, Wang J, Chen LL, et al. Application of fluorescent imaging system combined with methylene blue in sentinel lymph node biopsy of early breast cancer. Medical Journal of Communications 2019;33:279-80.
- Bai HY, Liu HM, Yang P, et al. Analysis of the Value of Indocyanine Green Tracer in Sentinel Lymph Node Biopsy of Breast Tumor. Chinese Journal of Minimally Invasive Surgery 2020;20:14-8.
- Huang R, Wu W, Ou L, et al. Application of Idocyanine green Combined with Methylene Blue in Sentinel Lymph Node Biopsy of Breast Cancer. Medical Innovation of China 2020;17:140-3.
- Zhang C, Li Y, Wang X, et al. Clinical study of combined application of indocyanine green and methylene blue for sentinel lymph node biopsy in breast cancer. Medicine (Baltimore) 2021;100:e25365. [Crossref] [PubMed]
- Fang L, Wang XZ, Liu ZY, et al. Comparative study ofmethylene blue tracer and double tracer containing nuclide in sentinel lymph node biopsy of breast cancer. Zhonghua Zhong Liu Za Zhi 2021;43:213-7. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. V.4. 2020. Available online:https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Lyman GH, Somerfield MR, Bosserman LD, et al. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:561-4. [Crossref] [PubMed]
- Guidelines and norms for breast cancer diagnosis and treatment by the China Anti-Cancer Association (2019 edition). Chinese Journal of Cancer 2019;29:609-80.
- Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-20. [Crossref] [PubMed]
- Eldrageely K, Vargas MP, Khalkhali I, et al. Sentinel lymph node mapping of breast cancer: a case-control study of methylene blue tracer compared to isosulfan blue. Am Surg 2004;70:872-5. [PubMed]
- Li J, Chen X, Qi M, et al. Sentinel lymph node biopsy mapped with methylene blue dye alone in patients with breast cancer: A systematic review and meta-analysis. PLoS One 2018;13:e0204364. [Crossref] [PubMed]
- Wang J, Wu R, Liu X, et al. Application value of dual-tracer method of indocyanine green plus methylene blue in sentinel lymph node biopsy of breast cancer in China: a Meta-analysis. Chinese Journal of General Surgery 2020;29:532-42.
- Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer 2006;106:4-16. [Crossref] [PubMed]
- Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994;220:391-8; discussion 398-401. [Crossref] [PubMed]
- Hung WK, Chan CM, Ying M, et al. Randomized clinical trial comparing blue dye with combined dye and isotope for sentinel lymph node biopsy in breast cancer. Br J Surg 2005;92:1494-7. [Crossref] [PubMed]
- Radovanovic Z, Golubovic A, Plzak A, et al. Blue dye versus combined blue dye-radioactive tracer technique in detection of sentinel lymph node in breast cancer. Eur J Surg Oncol 2004;30:913-7. [Crossref] [PubMed]
- He PS, Li F, Li GH, et al. The combination of blue dye and radioisotope versus radioisotope alone during sentinel lymph node biopsy for breast cancer: a systematic review. BMC Cancer 2016;16:107. [Crossref] [PubMed]
- Buchholz TA, Strom EA, McNeese MD, et al. Radiation therapy as an adjuvant treatment after sentinel lymph node surgery for breast cancer. Surg Clin North Am 2003;83:911-30. x. [Crossref] [PubMed]
- Wong SL, Edwards MJ, Chao C, et al. Sentinel lymph node biopsy for breast cancer: impact of the number of sentinel nodes removed on the false-negative rate. J Am Coll Surg 2001;192:684-9; discussion 689-91. [Crossref] [PubMed]
- Guo BL, Li T, Liu YH, et al. Sentinel lymph node biopsy expert consensus and technical operation guidelines for early breast cancer (2018 edition). Chinese Journal of Practical Surgery 2018;38:855-8.



