
The trends and efficacy of operation in the treatment of hepatocellular carcinoma
Introduction
The incidence of hepatocellular carcinoma (HCC) becomes more frequent worldwide, with a 75% increase in the newly diagnosed cases from 1990 to 2015. It has been predicted that by 2030, HCC may become the third leading cause of cancer-related deaths in the USA (1). The recommendations for the first-line treatment of early HCC include liver resection, transplantation or ablation. The first-line therapy for intermediate stages of HCC is transarterial chemoembolization (TACE), which can improve two-year survival outcomes. In advanced HCC with the tumor invading blood vessels and metastasizing to regional lymph nodes or distant organs, molecular-targeted drugs (sorafenib or lenvatinib) are recommended as the treatment of choice, which can extend the survival of the patients by up to 4 months (1,2).
Drug-eluting beads, as a new embolic material, have been developed. The drug-eluting beads may reduce complications of the TACE and prolong the progress-free survival but not overall survival (OS) (3,4). However, some studies have documented that patients with intermediate HCC may have better survival after surgery than TACE (5-7). A prospective randomized controlled trial (RCT) showed that the 1-, 2-, and 3-year OS rates of patients with resectable multiple HCC outside of Milan Criteria who received surgery were higher than that those of patients treated with TACE (P<0.001) (5). Another meta-analysis indicated that patients with intermediate-advanced HCC (Barcelona Clinic Liver Cancer, BCLC B/C) who were treated by surgery experienced greater survival benefit than those who received TACE therapy. In fact, all patients in the surgery group had longer OS than patients in the TACE group (P<0.001) (8). However, these studies had certain limitations. An insufficient number of patients were included in both investigations, and the meta-analysis included only one RCT, which might have led to a low level of evidence. The operation and surgery rate of patients are still unclear.
In view of these uncertainties, the present population-based study aimed to analyze the trends of operation (including surgery, transplantation, and ablation) and surgery, and to identify the optimal type of treatment for patients with different subgroups. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1551/rc).
Methods
Study cohort
Using Surveillance, Epidemiology, and End Results Program (SEER) 18 registries database, patients, 35–84 years old, diagnosed with HCC [International Classification of Diseases for Oncology, Third Edition (ICD-O-3), histology code 8170-8175, site code C220 (liver)] between 2004 and 2015 were included. Patients for which the information on whether they received operation was not available, were excluded. This study was conducted in accordance with the Helsinki Declaration (as revised in 2013). The college ethics committee approved the retrospective study. The requirement to obtain informed consent was waived by the institutional review board due to population-based study.
Statistical analysis
The patients’ information was extracted from the SEER database using the SEER*Stat software (version 8.3.5). Temporal trend of the rates of operation, surgery and non-operation for the treatment of HCC was estimated using Cochran-Armitage test (9). Median overall survival (mOS) was determined using Kaplan-Meier survival curves and was compared by log-rank test (10). The prognostic factors for all patients and the surgery group were assessed by Cox proportional hazards model (11).
The characteristics of gender, ethnicity, age of diagnosis, year of diagnosis, tumor status, American Joint Committee on Cancer (AJCC) stage, tumor size, tumor numbers, whether they received radiotherapy and whether they received chemotherapy were included into propensity score matching (PSM) analysis. The optimal caliper was set as 0.00001 and 9,436 pairs of were matched by 1:1 nearest neighbor approach. All statistical tests were two-sided, and the α level of P<0.05 was considered statistically significant. SPSS v24.0 (IBM, Chicago, IL, USA) and SAS 9.4 statistical software (SAS Institute, Cary, NC, USA) were used to perform all statistical analyses.
Definitions of tumor status
Localized tumor is defined as the tumor is confined to the liver and does not invade regional lymph nodes or metastases to distant tissue. Regional tumor is defined as the tumor invades the regional tissue. Distant tumor is defined as the tumor metastases to distant organs or invades distant lymph nodes.
Results
A total of 64,019 patients were included, including 15,746 who received operation, and 48,273 who did not receive operation.
For the survival analysis, patients with survival months code 0 (contact lost after diagnosis) and 9999 (unknown length of survival month) and patients with transplantation were excluded, leaving 52,338 patients in this part of the study. After PSM, a total of 18,872 patients were included into survival analysis. Among them, 9,436 patients received operation treatment and 9,436 patients received non-operation treatment. The baseline characteristics of patients before PSM and after PSM in the two groups were compared (Table 1).
Table 1
| Characteristics | Before PSM (N=52,338) | After PSM (N=18,872) | |||||
|---|---|---|---|---|---|---|---|
| Operation (N=12,348) | Non-operation (N=39,990) | P value | Operation (N=9,436) | Non-operation (N=9,436) | P value | ||
| Gender | <0.001 | 0.729 | |||||
| Male | 9,180 | 31,292 | 7,272 | 7,292 | |||
| Female | 3,168 | 8,698 | 2,164 | 2,144 | |||
| Ethnicity | <0.001 | 0.633 | |||||
| White | 7,888 | 27,555 | 6,657 | 6,604 | |||
| Black | 1,452 | 5,771 | 1,071 | 1,108 | |||
| Other | 3,008 | 6,664 | 1,708 | 1,724 | |||
| Age of diagnosis | <0.001 | 0.808 | |||||
| 35–44 | 340 | 794 | 162 | 146 | |||
| 45–54 | 1,905 | 6,873 | 1,499 | 1,484 | |||
| 55–64 | 4,765 | 15,334 | 3,740 | 3,751 | |||
| ≥65 | 5,338 | 16,989 | 4,035 | 4,055 | |||
| Year of diagnosis | <0.001 | 0.942 | |||||
| 2004–2007 | 3,406 | 9,217 | 2,581 | 2,560 | |||
| 2008–2011 | 3,869 | 13,689 | 2,949 | 2,961 | |||
| 2012–2015 | 5,073 | 17,084 | 3,906 | 3,915 | |||
| Tumor status | <0.001 | 0.981 | |||||
| Localized | 9,450 | 17,475 | 6,902 | 6,907 | |||
| Regional | 2,225 | 12,126 | 1,946 | 1,945 | |||
| Distant | 360 | 6,558 | 303 | 294 | |||
| Unknown | 313 | 3,831 | 285 | 290 | |||
| AJCC stage | <0.001 | 0.980 | |||||
| I | 6,808 | 11,220 | 4,959 | 4,973 | |||
| II | 2,900 | 6,227 | 2,188 | 2,167 | |||
| III | 1,604 | 9,033 | 1,384 | 1,371 | |||
| IV | 297 | 6,313 | 271 | 274 | |||
| Unknown | 739 | 7,197 | 634 | 651 | |||
| Tumor size (cm) | <0.001 | 0.983 | |||||
| ≤5 | 8,062 | 16,535 | 6,110 | 6,116 | |||
| 5–7 | 1,455 | 5,209 | 1,085 | 1,062 | |||
| 7–9 | 717 | 3,376 | 519 | 529 | |||
| >9 | 1,336 | 6,038 | 1,018 | 1,017 | |||
| Unknown | 778 | 8,832 | 704 | 712 | |||
| Tumor number | 0.704 | ||||||
| 1 | 9,807 | 34,191 | <0.001 | 7,994 | 7,978 | ||
| 2 | 2,042 | 4,879 | 1,248 | 1,268 | |||
| 3 | 384 | 760 | 163 | 167 | |||
| >3 | 115 | 160 | 31 | 23 | |||
| Radiotherapy | <0.001 | 0.925 | |||||
| Yes | 385 | 3,784 | 284 | 278 | |||
| No | 11,940 | 36,093 | 9,147 | 9,152 | |||
| Unknown | 23 | 113 | 5 | 6 | |||
| Chemotherapy | <0.001 | 0.668 | |||||
| Yes | 3,540 | 19,173 | 3282 | 3,254 | |||
| No | 8,808 | 20,817 | 6154 | 6,182 | |||
PSM, propensity score matching.
To determine whether patients could gain an additional survival benefit from surgery, the patients were divided into seven groups (group A–G) based on the BCLC stage A and B. Group A included patients with a single tumor size smaller than 5 cm. Group B included patients with a single tumor larger than 5 cm but no more than 7 cm. Group C included patients with a single tumor larger than 7 cm but no more than 9 cm. Group D included patients with a single tumor larger than 9 cm. Group E included patients with 2–3 tumors no more than 3 cm. Group F included patients with 2–3 tumors larger than 3 cm. Group G included patients with multiple tumors (>3 tumors) (Figure 1).
Trends of operation and surgery on HCC
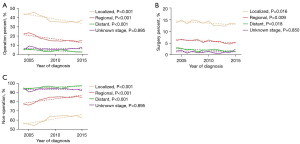
The trends of operation rate increased in localized, regional and distant group (all P<0.001). (Figure 2A). For surgery, the frequencies and trends in localized (P=0.016), regional (P=0.009), distant group (P=0.018) declined with time (Figure 2B). For patients without operation treatment, the non-operation rate in the localized, regional and distant group increased (all P<0.001) (Figure 2C).
mOS in all patients and the subgroups
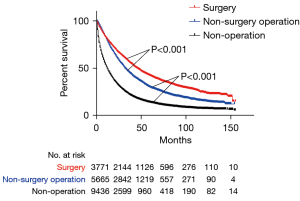
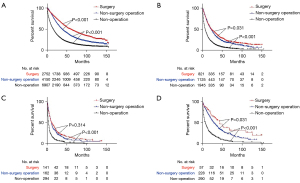
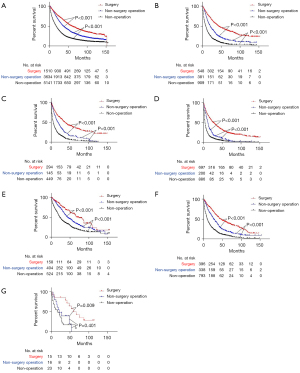
After PSM, the mOS of patients in the surgery group (45 months, 95% CI: 42.4–47.6) was longer than that in the non-surgical procedure group (32 months, 95% CI: 31.1–32.9, P<0.001), which was longer than that in the non-operation group (12 months, 95% CI: 11.7–12.3), P<0.001) (Figure 3). The mOS of patients in localized group receiving surgery (59 months, 95% CI: 54.8–63.2) was longer than that of patients with non-surgical procedure (38 months, 95% CI: 36.1–39.9, P<0.001), which was longer than patients with non-operation (16 months, 95% CI: 15.3–16.7, P<0.001) (Figure 4A). Similar results were presented in reginal, distant and unknown stage groups. In reginal group, the mOS of patients with surgery (23 months, 95% CI: 19.9–26.1) was longer than patients with non-surgical procedure (22 months, 95% CI: 20.1–23.9, P=0.031), which was longer than patients in the non-operation (7 months, 95% CI: 6.4–7.6, P<0.001) (Figure 4B). In distant group, the mOS of patients with non-surgical procedure (11 months, 95% CI: 8.9–13.1) was longer than patients with non-operation (4 months, 95% CI: 3.5–4.5, P<0.001) (Figure 4C). In the unknown stage group, the patients with surgery had longer mOS than patients with non-surgery operation and patients without operation (both P<0.05) (Figure 4D). In the subgroups analysis, the patients in the group A who underwent surgery (59 months, 95% CI: 53–65) had better survival than patients with non-surgical procedure (36 months, 95% CI: 34.1–37.9, P<0.001), which was longer than patients with non-operation (18 months, 17.1–18.9, P<0.001) (Figure 5A). In group B, patients with surgery (43 months, 95% CI: 36–50) had also better survival than patients with non-surgical procedure (21 months, 95% CI: 18–24, P<0.001), which was longer than patients with non-operation (9 months, 95% CI: 8–10, P<0.001) (Figure 5B). In group C, the mOS of patients with surgery (37 months, 95% CI: 29.9–44.1) was longer than patients with non-surgical procedure (19 months, 95% CI: 14–24, P<0.001), which was longer than patients with non-operation (6 months, 95% CI: 4.9–7.1, P<0.001) (Figure 5C). In group D, the mOS of patients with surgery (27 months, 95% CI: 23.2–30.8) was longer than patients with non-surgical procedure (12 months, 95% CI: 10.4–13.6, P<0.001), which was longer than patients in the non-operation (4 months, 95% CI: 3.7–4.3, P<0.001) (Figure 5D). In group E, the mOS of patients with surgery (74 months, 95% CI: 56.6–91.4) was longer than patients with non-surgical procedure (44 months, 95% CI: 36.9–51.1, P<0.001), which was longer than patients with non-operation (23 months, 95% CI: 20.2–25.8, P<0.001) (Figure 5E). In group F, the mOS of patients with surgery (47 months, 95% CI: 39.1–54.9) was longer than patients with non-surgical procedure (27 months, 95% CI: 23.6–30.4, P<0.001), which was longer than patients with non-operation (10 months, 95% CI: 8.8–11.2, P<0.001) (Figure 5F). In group G, the mOS of patients with surgery (67 months, 95% CI: 43.6–90.4) was longer than patients with non-surgical procedure (22 months, 95% CI: 6.8–37.2, P=0.009) (Figure 5G).
Predictors of OS in all patients and the operation group
The multivariable regression analysis of all patients showed that operation was an independent favor factor for OS compared with non-operation (HR: 2.414, 95% CI: 2.329–2.499, P<0.001) for all patients after PSM. For patients with operation, the multivariable regression analysis showed that surgery was an independent favor factor for OS compared with patients with non-surgical procedure (HR: 1.556, 95% CI: 1.471–1.667, P<0.001) (Table 2).
Table 2
| Characteristics | All patients | Patients in the operation group | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | |||||
| Male | Reference | Reference | |||
| Female | 0.947 (0.908, 0.987) | 0.010 | 0.924 (0.867, 0.985) | 0.015 | |
| Ethnicity | |||||
| White | Reference | Reference | |||
| Black | 1.077 (1.021, 1.136) | 0.006 | 1.129 (1.040, 1.226) | 0.004 | |
| Other | 0.769 (0.734, 0.833) | <0.001 | 0.747 (0.696, 0.802) | <0.001 | |
| Age of diagnosis | |||||
| 35–44 | Reference | Reference | |||
| 45–54 | 1.173 (1.018, 1.351) | 0.027 | 1.135 (0.924, 1.393) | 0.228 | |
| 55–64 | 1.158 (1.009, 1.330) | 0.037 | 1.123 (0.919, 1.372) | 0.256 | |
| ≥65 | 1.418 (1.235, 1.627) | <0.001 | 1.413 (1.157, 1.725) | 0.001 | |
| Year of diagnosis | |||||
| 2004–2007 | Reference | Reference | |||
| 2008–2011 | 0.869 (0.834, 0.906) | <0.001 | 0.833 (0.783, 0.886) | <0.001 | |
| 2012–2015 | 0.797 (0.763, 0.833) | <0.001 | 0.689 (0.643, 0.739) | <0.001 | |
| Tumor status | |||||
| Localized | Reference | Reference | |||
| Regional | 1.326 (1.259, 1.397) | <0.001 | 1.291 (1.194, 1.397) | <0.001 | |
| Distant | 1.785 (1.337, 2.384) | <0.001 | 1.620 (1.113, 2.358) | 0.012 | |
| Unknown | 0.939 (0.828, 1.064) | 0.324 | 1.055 (0.870, 1.279) | 0.585 | |
| AJCC stage | |||||
| I | Reference | Reference | |||
| II | 1.234 (1.186, 1.303) | <0.001 | 1.265 (1.178, 1.360) | <0.001 | |
| III | 1.479 (1.383, 1.581) | <0.001 | 1.623 (1.468, 1.794) | <0.001 | |
| IV | 1.470 (1.085, 1.993) | 0.013 | 1.742 (1.168, 2.597) | 0.006 | |
| Unknown | 1.377 (1.257, 1.508) | <0.001 | 1.223 (1.061, 1.409) | 0.005 | |
| Tumor size (cm) | |||||
| ≤5 | Reference | Reference | |||
| 5–7 | 1.403 (1.322, 1.488) | <0.001 | 1.211 (1.106, 1.327) | <0.001 | |
| 7–9 | 1.535 (1.421, 1.658) | <0.001 | 1.285 (1.140, 1.449) | <0.001 | |
| >9 | 1.889 (1.775, 2.009) | <0.001 | 1.612 (1.465, 1.774) | <0.001 | |
| Unknown | 1.430 (1.329, 1.538) | <0.001 | 1.223 (1.093, 1.369) | <0.001 | |
| Tumor number | |||||
| 1 | Reference | Reference | |||
| 2 | 0.898 (0.854, 0.945) | <0.001 | 0.913 (0.844, 0.987) | 0.021 | |
| 3 | 0.882 (0.774, 1.005) | 0.059 | 0.928 (0.759, 1.136) | 0.469 | |
| >3 | 0.879 (0.647, 1.196) | 0.413 | 0.681 (0.594, 1.405) | 0.681 | |
| Radiotherapy | |||||
| Yes | Reference | Reference | |||
| No | 1.112 (1.006, 1.229) | 0.038 | 0.812 (0.701, 0.940) | 0.005 | |
| Unknown | 1.073 (0.532, 2.165) | 0.843 | 0.530 (0.169, 1.668) | 0.278 | |
| Chemotherapy | |||||
| Yes | Reference | Reference | |||
| No | 1.393 (1.343, 1.445) | <0.001 | 0.968 (0.915, 1.024) | 0.252 | |
| Treatment | – | – | |||
| Operation | Reference | – | – | ||
| Non-operation | 2.414 (2.329, 2.499) | <0.001 | – | – | |
| Treatment | – | – | |||
| Surgery | – | – | Reference | ||
| Non-surgery operation | – | – | 1.556 (1.471, 1.667) | <0.001 | |
PSM, propensity score matching; AJCC, American Joint Committee on Cancer.
Discussion
The incidence of HCC in men and women increased from 2000 to 2013 and is predicted to increase until 2030 (12). The main finding was that the trends of operation and surgery rate in the localized, regional, and distant group declined from 2004 to 2015. And the survival of patients with localized, regional, distant tumor, single tumor larger than 5 cm, 2–3 tumors larger than 3 cm and multiple tumors (>3 tumors) benefits more from surgery than non-surgical procedure.
Since 1999, BCLC criteria have been used as a recommended guide for HCC treatment (13). Surgery was advised for the treatment of HCC patients with BCLC stage A, who had good liver function and good physical condition. This recommendation limited the application of surgery in HCC treatment. For patients with BCLC stage B, TACE was recommended as the first-line treatment. HCC patients with BCLC stage B who received TACE treatment had shorter mOS (16 to 42 months) than patients undergoing surgery (23 to 70 months) (6,14-18). However, HCC patients with TACE have high recurrence due to incomplete embolization (19,20). Alternatively, the tumor can be removed completely by liver resection, reducing tumor recurrence and metastasis. It was suggested that patients with a single tumor larger than 5 cm should be classified as BCLC stage A and receive a better survival benefit from liver resection (21). In the present analysis, patients were divided into seven groups according to the size and number of tumors. The mOS of HCC patients undergoing surgery in the group A–G surgery was the longest. Although the results of the study showed that patients with single tumor larger than 5 cm could get more survival from surgery than patients with non-surgical procedure. Thus far no RCT was performed for patients with single tumor larger than 5 cm that would focus on the effect of tumor size on the therapeutic efficacy of surgery or other types of operation, such as TACE. Therefore, the outcome of various treatments in patients with different tumor size was worth exploring by a large cohort study.
Multivariate analysis of patients with operation treatment also demonstrated that surgical treatment resulted in better mOS of the patients than non-surgical procedure. In agreement with earlier publications, older patients, male patients, black patients, patients with larger tumor size, patients with tumor metastasis, single patients and patients with an early diagnosis of HCC had a worse prognosis. Several studies have shown that a higher number of tumors is associated with a worse prognosis of the HCC patients with radical treatment (22-24). However, the current work documented that the presence of two tumors increased the mOS of patients. That might be the reason that patients with single tumor who were worse than patients with two tumors (AJCC stage III and IV: 18% vs. 15%).
Although this study provided encouraging results for patients treated with operation, in particular by surgery, the operation rate did not increase significantly with time, and the surgery rate decreased. These trends may be explained by the emergence of new drugs, such as sorafenib. The recommendation by the BCLC criteria that patients with BCLC stage A and good liver function and physical condition should be treated by surgery might be another reason for the decline in the treatment of HCC by surgery. However, the results of the present analysis support the notion that patients can obtain a better survival benefit from surgery than from other types of operations.
The retrospective design of the present study constitutes its limitation since it might have led to a selection bias. However, the analysis was based on a large number of patients, which could increase the reliability of the results. The efficacy of surgery was compared only with non-surgical procedure and non-operation treatments, but comparisons with specific treatments were not performed. While this approach might not provide strong proof that surgery is the best treatment for patients with a localized tumor or regional metastases, the evidence was obtained that patients can receive a better survival benefit from operation, and surgery might be the best treatment choice. This study did not answer the question of whether patients with poor liver function and physical status should be treated surgically, but from the side, these patients could get better survival after undergoing operation. The conclusions reached here need to be confirmed with a RCT in the future.
Conclusions
In conclusion, this study provided a comprehensive analysis of the trends in the rate of operation and surgery for the treatment of HCC. Moreover, the efficacy of surgery was compared with the efficacy of non-surgical procedure and non-operation in patients with different subgroups of HCC. The results indicated that patients with single tumor larger than 5 cm or patients with 2–3 tumors larger than 3 cm might get more survival from surgery than patients with non-surgical procedures or non-operation.
Acknowledgments
We acknowledge that SEER database provided the data used in the study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1551/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1551/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Helsinki Declaration (as revised in 2013). The college ethics committee approved the retrospective study. The requirement to obtain informed consent was waived by the institutional review board due to population-based study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:541-51. [Crossref] [PubMed]
- Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82-8. [Crossref] [PubMed]
- Tada T, Kumada T, Toyoda H, et al. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: A multicenter study from Japan. Cancer Sci 2017;108:1414-20. [Crossref] [PubMed]
- Lee JM, Jang BK, Lee YJ, et al. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin Mol Hepatol 2016;22:160-7. [Crossref] [PubMed]
- Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology 2018;68:977-93. [Crossref] [PubMed]
- Buonaccorsi JP, Laake P, Veierød MB. On the power of the Cochran-Armitage test for trend in the presence of misclassification. Stat Methods Med Res 2014;23:218-43. [Crossref] [PubMed]
- van Walraven C, McAlister FA. Competing risk bias was common in Kaplan-Meier risk estimates published in prominent medical journals. J Clin Epidemiol 2016;69:170-3.e8. [Crossref] [PubMed]
- Staley JR, Jones E, Kaptoge S, et al. A comparison of Cox and logistic regression for use in genome-wide association studies of cohort and case-cohort design. Eur J Hum Genet 2017;25:854-62. [Crossref] [PubMed]
- Petrick JL, Kelly SP, Altekruse SF, et al. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34:1787-94. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Kim JY, Sinn DH, Gwak GY, et al. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol Hepatol 2016;22:250-8. [Crossref] [PubMed]
- Lin CW, Chen YS, Lo GH, et al. Comparison of overall survival on surgical resection versus transarterial chemoembolization with or without radiofrequency ablation in intermediate stage hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol 2020;20:99. [Crossref] [PubMed]
- Luo J, Peng ZW, Guo RP, et al. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology 2011;259:286-95. [Crossref] [PubMed]
- Lin CT, Hsu KF, Chen TW, et al. Comparing hepatic resection and transarterial chemoembolization for Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma: change for treatment of choice? World J Surg 2010;34:2155-61. [Crossref] [PubMed]
- Naar L, Hatzaras I. Liver Resection for Hepatocellular Carcinoma and the Barcelona Clinic Liver Cancer Criteria: Is It Time to Push the Limits? Ann Surg Oncol 2020;27:2122-4. [Crossref] [PubMed]
- Zhang J, Zhang Q, Lou Y, et al. Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology 2018;67:1872-89. [Crossref] [PubMed]
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci 1999;24:68-72. [Crossref] [PubMed]
- Forner A, Gilabert M, Bruix J, et al. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol 2014;11:525-35. [Crossref] [PubMed]
- Toyoda H, Kumada T, Kaneoka Y, et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol 2008;49:223-32. [Crossref] [PubMed]
- Toyoda H, Kumada T, Tada T, et al. Prognostic significance of a combination of pre- and post-treatment tumor markers for hepatocellular carcinoma curatively treated with hepatectomy. J Hepatol 2012;57:1251-7. [Crossref] [PubMed]
- Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg 2019;154:209-17. [Crossref] [PubMed]



