
Metastatic papillary thyroid carcinoma with no primary tumor in the thyroid gland: a case report and review of literature
Introduction
Papillary thyroid cancer is the most common and curable among all the types of thyroid malignancies. It usually presents as a palpable or unpalpable thyroid mass or nodule. Incidental ultrasound discovery of thyroid nodules is not uncommon. Papillary thyroid carcinomas can metastasize to the cervical lymph nodes and other distant sites. Thyroid carcinoma with lymph node papillary carcinoma metastasis but without a primary thyroid lesion is a rare phenomenon. This article reports a case where metastatic papillary thyroid carcinoma was found in the cervical lymph nodes with no primary tumor in the thyroid gland. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1780/rc).
Case presentation
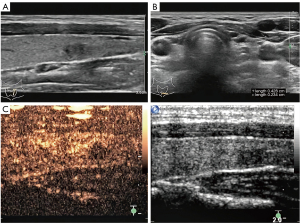
A 27-year-old female was diagnosed with a tiny nodule in her left thyroid gland, which was incidentally detected in an ultrasonographic examination. She presented without fever, weight loss, or night sweats. No palpable mass was found in the physical examination. Her family, medical, and social history was unremarkable. Thyroid ultrasonography revealed a hypoechoic nodule in the lower pole of the left lobe, with a size of about 0.4 cm × 0.3 cm, an irregular shape, an unclear boundary, an uneven internal echo, and no definite envelope-like echo. No obvious blood flow signal was observed in the nodule. Several enlarged lymph nodes were found below the lower left lobe, the largest of which was about 0.4 cm × 0.2 cm. The lymph nodes were regular in shape, with clear edges, hypoechoic in the periphery, and hyperechoic in the middle. No abnormal echo was found in the thyroid isthmus or the right lobe. We further examined the hypoechoic nodule using contrast-enhanced ultrasound and identified it as a thyroid imaging reporting and data system (TI-RADS) category 4b nodule (Figure 1). The preoperative blood routine, thyroid function, and thyroglobulin test results were normal. Needle aspiration cytology of the thyroid and lymph nodes was recommended. However, the patient was anxious and refused to have a fine-needle aspiration biopsy (FNAB), requesting surgical excision instead. She underwent a left thyroid lobectomy and ipsilateral central lymph node dissection under general anesthesia. The thyroid specimen was cut open, and a nodule (0.4 cm × 0.3 cm in size) located in the middle part of the thyroid was visible to the naked eye. The nodule was solid and regularly shaped with a slightly hard texture, a relatively clear boundary, and a complete capsule. The thyroid lobes and central lymph nodes were sent for intraoperative frozen section and pathological examination. The results of this examination stated that the thyroid nodules were considered benign but that confirmation by paraffin section was required and that central lymph node metastasis was suspected. The incision was then closed and the operation ended.
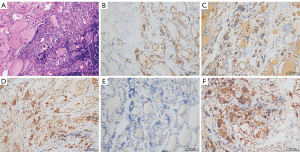
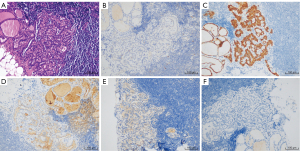
A week later, we received the final pathological and immunohistochemical results of the paraffin section. Two endocrine pathologists had examined the paraffin section carefully and found no detectable primary tumor despite a full histopathologic examination of the left thyroid gland. The thyroid nodule showed local fibrous hyperplasia with atypical cell projections. The immunohistochemical study of the nodule was negative to cytokeratin 19, positive to thyroglobulin, negative to cluster of differentiation (CD) 56, negative to galectin-3, and positive to the thyroid peroxidase antibody (Figure 2). Metastasis was found in 2 of the 15 central lymph nodes with the following immunohistochemistry: positive to cytokeratin 19, positive to thyroglobulin, negative to CD 56, positive to galectin-3, and negative to the thyroid peroxidase antibody (Figure 3). According to the tumor, node, and metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC), the patient’s thyroid carcinoma stage was TxN1aM0. Further surgery to remove the right thyroid gland was recommended, but the patient refused, opting instead for observation and follow-up. Clinical physical examination, ultrasound, computed tomography (CT), and blood examinations were used to follow up the patient for 2 years. No tumor recurrence or metastasis was found, and no nodules were observed in the right thyroid gland. The patient recovered well without complications and was satisfied with the treatment and outcome. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Occult thyroid carcinoma (OTC) was usually defined as an impalpable thyroid carcinoma, generally smaller than 1.0 cm in diameter (1). The World Health Organization (WHO) disease classification system defines OTC as an incidental disease, and it is called occult because it is usually detected at autopsy or during secondary surgery (2). Boucek et al. classified OTC into four different types (3). The first type includes patients with benign thyroid disease who are incidentally diagnosed with thyroid cancer after a total thyroidectomy (TT) or at autopsy. The second type includes patients with papillary microcarcinoma of the thyroid that is found incidentally in imaging tests, such as ultrasound. The third type includes patients with clinically metastatic thyroid cancer, where the primary tumor is undetectable before surgery but is eventually found in histological specimens. The fourth type includes patients with thyroid cancer localized in ectopic thyroid tissue. Liu et al. presented the fifth type of OTC in which a thyroid gland lesion is diagnosed as benign according to pathological and imaging evaluations, but metastases of a thyroid carcinoma are detected in either locoregional lymph nodes or distant organs (4). Patients in this fifth category were further classified into two groups. In the first, metastases of a thyroid carcinoma is detected in locoregional lymph nodes. In the second type, a distant organ metastatic mass is detected and diagnosed as metastasis from a thyroid carcinoma. Our patient fits into the first group of the fifth type of OTC.
According to the Chinese guidelines for the diagnosis and treatment of thyroid nodules and differentiated thyroid cancers (5), preoperative FNAB can help reduce unnecessary thyroid nodule surgery and help determine the surgical plan. Therefore, we recommended FNAB for our patient. However, the patient was very anxious and refused the FNAB, requesting surgical treatment. The guidelines also indicate that FNAB should be considered for thyroid nodules larger than 1 cm in diameter but can be avoided if there is a high suspicion of malignant nodules after ultrasound imaging. For thyroid nodules less than 1 cm in diameter, a routine FNAB is not recommended, but ultrasound-guided FNAB can be considered if signs of malignant nodules are indicated by ultrasound imaging. The diameter of the thyroid nodules in our patient was less than 1 cm, and the ultrasound image indicated malignancy in the thyroid nodules. Therefore, FNAB was not a mandatory recommendation. According to the 2020 Chinese guidelines for ultrasound risk stratification of thyroid nodules (6), 70.4% of surgeons reported that less than half of their thyroid surgery patients had undergone FNAB before surgery due to the limitations of FNAB technology and other practical concerns, such as cultural traditions, patient wishes, population mobility, and medical liability concerns. Therefore, many thyroid operations in China are not based on FNAB results, but only on ultrasound reports or other relevant clinical evidence. The guidelines also point out that the overdiagnosis and overtreatment of papillary thyroid microcarcinoma (PTMC) caused by the widespread use of ultrasound is concerning (6). Moreover, the expert consensus on the diagnosis and treatment of thyroid micropapillary carcinoma in China, released in 2016, also acknowledged that controversy exists regarding whether intraglandular PTMC (especially under 5 mm in diameter) can be closely follow up without surgery (7). Surgical decision should be made after comprehensive analysis of the clinical stage of the tumor, risk assessment, and full communication with patients and their families. For patients with a heavy psychological burden, surgical treatment can be considered. In our case, after communication with the patient, we respected the patient’s opinion and decided on surgical treatment without FNAB. During the operation, the left thyroid lobule was excised first, and 2 hard nodules with unclear boundaries from the surrounding adipose tissue were found in the lymph node beside the thyroid gland. Considering that the sensitivity of preoperative ultrasound to assess lymph node metastasis has been reported to range from 10.9% to 30% (8), we performed a lymph node resection. Unfortunately, an intraoperative frozen section could not confirm a suspected papillary carcinoma metastasis to the lymph nodes. As a precaution, a preventive central lymph node dissection was performed. Postoperative paraffin sections and immunohistochemistry confirmed that 2 of the 15 lymph nodes had papillary carcinoma metastasis.
There are several possible hypotheses that could explain why a papillary thyroid carcinoma would metastasize without a primary tumor in the thyroid gland. The first possible reason for the missed diagnosis is that the pathologist did not open all the thyroid tissue samples layer by layer and carefully examine them in accordance with the regulations, thereby missing the tumor tissue. If a lesion is smaller than 3 mm, it might be missed. Therefore, we should focus on the selection of fibrous scar tissue or gray nodules. For calcified specimens, soft tissue structures around the calcified tissues can be cut, and conventional paraffin section processing can be performed after decalcification of the calcified tissues, but the integrity of the cell structure should be maintained.
Another hypothesis is tumor regression. Spontaneous regression of a tumor is defined as the partial or total disappearance of a tumor when it has not been treated at all, or when it has not been treated enough to affect the systemic process of the tumor. The estimated incidence of tumor regression is about 1 in 140,000 (9). The most common malignancies with spontaneous regression are neuroblastoma, choriocarcinoma, renal carcinoma, and malignant melanoma, which have been well reported in the literature (10-13). By studying 176 cases of the spontaneous regression of cancer, Cole found that stimulation of the immune process is the most important factor in the spontaneous regression of cancer. There are many stimulating factors, including bacterial products, enzymes, infection, hormones, and trauma (14). The immune system also plays an important role in the clinical evolution of papillary thyroid carcinoma. In papillary thyroid carcinoma, phagocytosis of tumor cells by macrophages has been identified and is associated with an increased incidence of lymph node metastasis, extra-thyroid infiltration, and distant metastasis (15). Thus, fibrosis may be a sign of partial or complete tumor regression in thyroid cancer or may be a cause of this rare phenomenon. In some cases where fibrosis is very extensive, tumor cells are rarely found (16). Simpson analyzed the histological and clinical features of 2 cases of thyroid papillary microcarcinoma, one with a diffuse sclerosis variant and the other with a multicellular follicular variant, both of which suggested partial tumor regression (17). Nishikawa et al. reported a case of primary occult papillary thyroid carcinoma with bone metastasis, but only diffuse dense fibrosis with lymphocytic infiltration was found in the thyroid gland, and no primary thyroid lesion was detected. It has been suggested that some occurrences of primary occult papillary thyroid carcinoma may subside or disappear after distant metastasis due to immunity or other host resistance factors (18).
In addition, papillary carcinoma may occur in ectopic thyroid tissue. An ectopic thyroid is a congenital developmental disorder. It can be located along the embryonic migration path of the thyroid gland, from the foramen cecum to the anterior mediastinum (19). Ectopic thyroid tissues located in the kidney, heart, gallbladder, and pancreas have also been reported (20). It is possible that carcinoma can develop in the ectopic thyroid tissue and then metastasize to the locoregional lymph nodes or distant organs while the thyroid itself remains normal. It has been reported that ectopic thyroid cancer can occur in the lateral neck area, mediastinum, and lungs (21-23).
Conclusions
We encountered an extremely rare case of lymph node thyroid carcinoma with no primary lesion. The possible explanations for this phenomenon include the tumor lesion being missed on pathologic biopsy, tumor regression, and ectopic thyroid carcinoma. It is necessary to understand these rare clinical and pathological manifestations to avoid missed diagnoses.
Acknowledgments
We would like to thank L. Roberts and J. Gray for their help in polishing our paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1780/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1780/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1780/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moosa M, Mazzaferri EL. Occult thyroid carcinoma. Cancer 1997;10:180-8.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 7th Edition. Berlin: Springer-Verlag, 1992.
- Boucek J, Kastner J, Skrivan J, et al. Occult thyroid carcinoma. Acta Otorhinolaryngol Ital 2009;29:296-304. [PubMed]
- Liu H, Lv L, Yang K. Occult thyroid carcinoma: a rare case report and review of literature. Int J Clin Exp Pathol 2014;7:5210-4. [PubMed]
- Gao M. Guidelines for the diagnosis and treatment of thyroid nodules and differentiated thyroid carcinoma. Chinese Journal of Clinical Oncology 2012;39:1249-72.
- Superficial Organ and Vascular Ultrasound Group, Society of Ultrasound in Medicine, Chinese Medical Association, Chinese Artificial Intelligence Alliance for Thyroid and Breast Ultrasound. 2020 Chinese Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules: The C-TIRADS. Chinese Journal of Ultrasonography 2021;30:185-200.
- Chinese Association of Thyroid Oncology. Chinese expert consensus on diagnosis and treatment of thyroid micropapillary Carcinoma (2016 edition). Chinese Journal of Clinical Oncology 2016;405-11.
- Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope 2011;121:487-91. [Crossref] [PubMed]
- Elston DM. Mechanisms of regression. Clin Med Res 2004;2:85-8. [Crossref] [PubMed]
- Cozzi DA, Mele E, Ceccanti S, et al. Long-term follow-up of the "wait and see" approach to localized perinatal adrenal neuroblastoma. World J Surg 2013;37:459-65. [Crossref] [PubMed]
- Hertz R. Spontaneous regression in choriocarcinoma and related gestational trophobalstic neoplasms. Natl Cancer Inst Monogr 1976;44:59-60. [PubMed]
- Williams T, Rodriguez R, Murray K, et al. Metastatic papillary renal cell carcinoma regression after cytoreductive nephrectomy. Urology 2015;85:283-7. [Crossref] [PubMed]
- Sumner WC, Foraker AG. Spontaneous regression of human melanoma. Clinical and experimental studies. Cancer 2015;13:79-81. [Crossref] [PubMed]
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol 1981;17:201-9. [Crossref] [PubMed]
- Baker JR Jr. The immune response to papillary thyroid cancer. J Clin Endocrinol Metab 1995;80:3419-20. [PubMed]
- Krassas GE, Pontikides NE. Werner and Ingbar's the Thyroid. 10th ed. Philadelphia: Lippincott Williams and Wilkins, 2013.
- Simpson KW, Albores-Saavedra J. Unusual findings in papillary thyroid microcarcinoma suggesting partial regression: a study of two cases. Ann Diagn Pathol 2007;11:97-102. [Crossref] [PubMed]
- Nishikawa M, Toyoda N, Yonemoto T, et al. Occult papillary thyroid carcinoma in Hashimoto's thyroiditis presenting as a metastatic bone tumor. Endocr J 1998;45:111-6. [Crossref] [PubMed]
- Oueslati S, Douira W, Charada L, et al. Ectopic thyroid. Ann Otolaryngol Chir Cervicofac 2006;123:195-8. [Crossref] [PubMed]
- Ibrahim NA, Fadeyibi IO. Ectopic thyroid: etiology, pathology and management. Hormones (Athens) 2011;10:261-9. [Crossref] [PubMed]
- Di Mari N, Barbagli L, Mourmouras V, et al. Ectopic thyroid of the lung. An additional case. Pathologica 2010;102:102-3. [PubMed]
- Shah BC, Ravichand CS, Juluri S, et al. Ectopic thyroid cancer. Ann Thorac Cardiovasc Surg 2007;13:122-4. [PubMed]
- Agosto-Vargas Y, Gutiérrez M, Martínez JH, et al. Papillary Thyroid Carcinoma: Ectopic Malignancy versus Metastatic Disease. Case Rep Endocrinol 2017;2017:9707031. [Crossref] [PubMed]


